24. Assertion :In B2H6, the terminal B H bonds are shorter, than
5 (750) · $ 9.00 · In stock
24. Assertion :In B2H6, the terminal B H bonds are shorter, than the B H bridge bonds Reason: The terminal B H bond order is greater than that of the B H bridge bond
24- Assertion-In B2H6- the terminal B-H bonds are shorter- than the B-H bridge bonds Reason- The terminal B-H bond order is greater than that of the B-H bridge bond

ChemicalBondingBYPMS PDF

The Source Function Descriptor as a Tool to Extract Chemical Information from Theoretical and Experimental Electron Densities
Why is bridge bond stronger but longer in diborane? - Quora

Identify correct order of bond angles (A) C120 > F20 and F20 AsBrz > AsCl3 (C) NO > NOZ vdrogen of B2H6 and Hy is the bridging (D) HBH, >H.BH,; where H

Geometrical Frustration of B-H Bonds in Layered Hydrogen Borides Accessible by Soft Chemistry - ScienceDirect

The Source Function Descriptor as a Tool to Extract Chemical Information from Theoretical and Experimental Electron Densities

A new look at the nido-undecaborate system - ScienceDirect
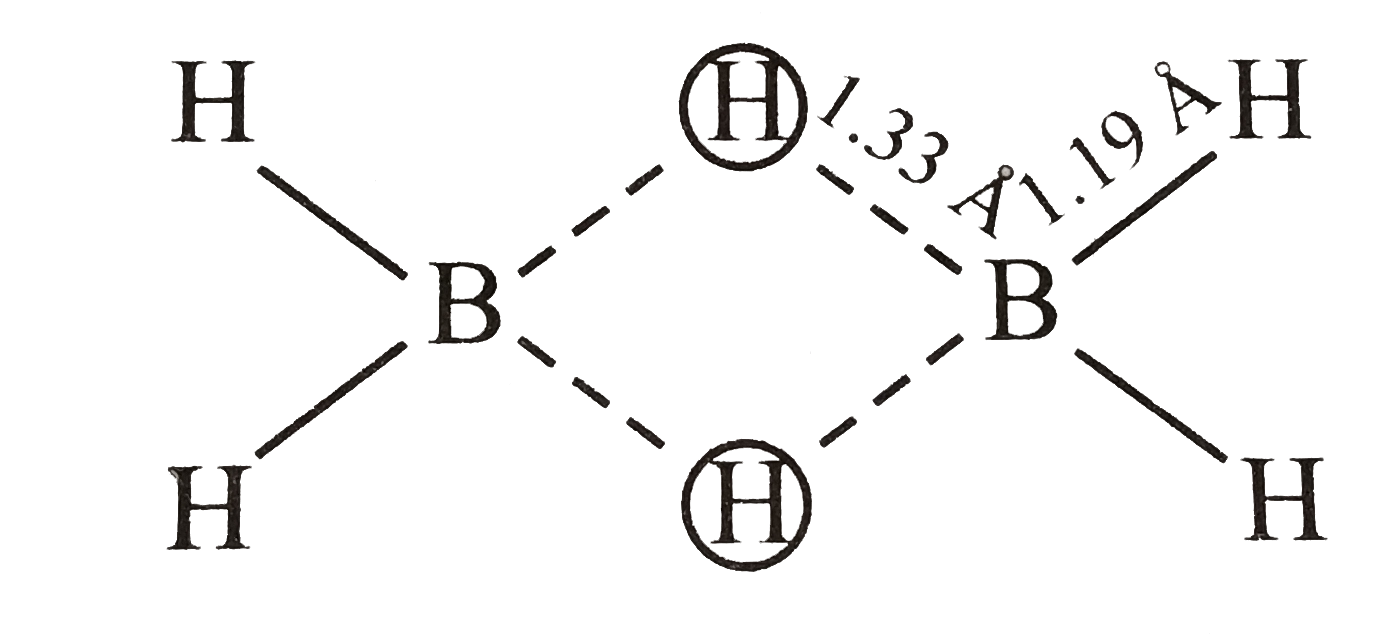
Consider the following statements, (i) Diborane contains two centre

Formation and Reactivity of Electron‐Precise B−B Single and Multiple Bonds - Arrowsmith - 2017 - Angewandte Chemie International Edition - Wiley Online Library

무기화학Solutions, PDF, Electron Configuration

EVERGREEN CHEM ADV by Innovartan - Issuu











