- Home
- cdefghi 42g
- 42g of N₂ react with excess of O₂ to produce NO. Amount of NO formed is a.60g b.32g c.45g d.90g
42g of N₂ react with excess of O₂ to produce NO. Amount of NO formed is a.60g b.32g c.45g d.90g
4.8 (233) · $ 16.50 · In stock
Share your videos with friends, family and the world

UMAIR KHAN ACADEMY
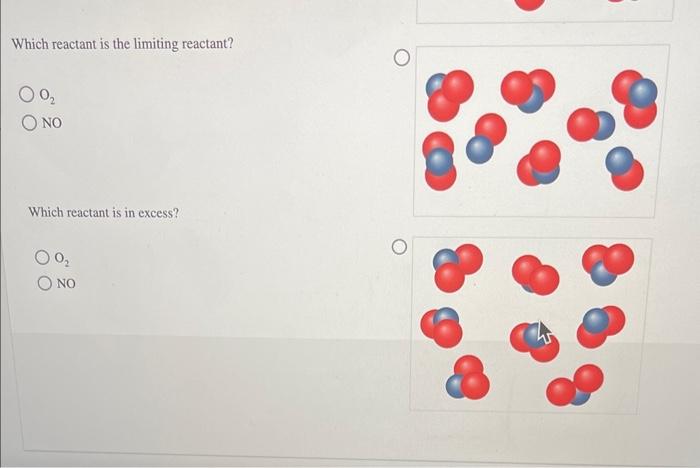
Solved reacts with NO to form NO2 according to the ollowing

Consider the reaction: NO2( g) ¡ NO( g) + 1 2 O2( g) The tabulate
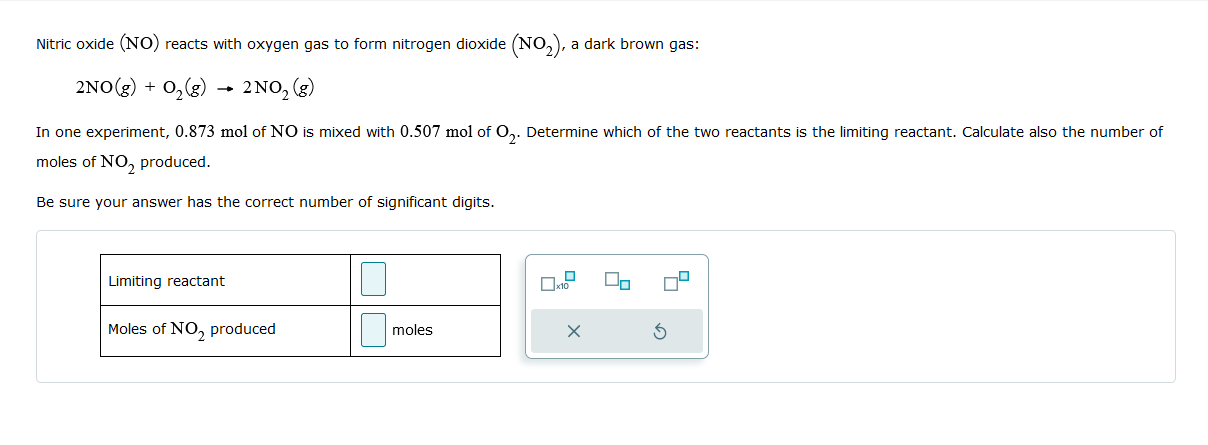
Solved Nitric oxide (NO) reacts with oxygen gas to form
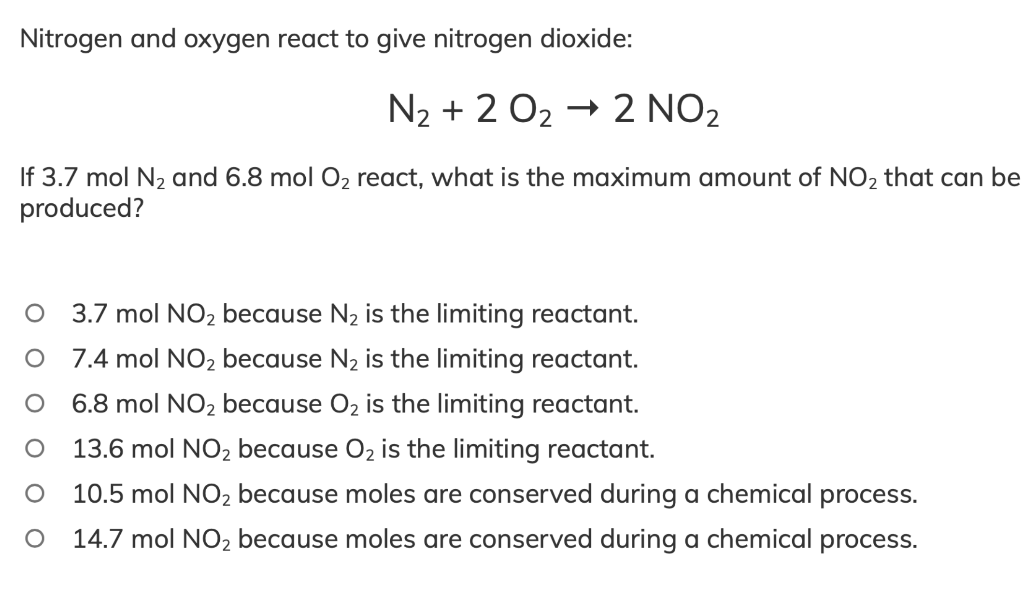
Solved Nitrogen and oxygen react to give nitrogen dioxide
42. If 25 g N2 reacts with H2 then amount of NH3 produced in
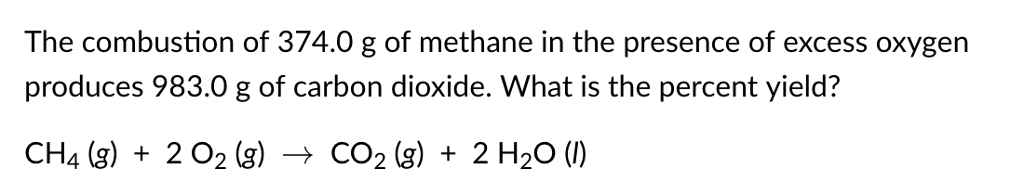
Solved If 42.0 g of nitrogen gas reacts with an excess of

UMAIR KHAN ACADEMY
Chemist2U) Priceline World Square restaurant menu in Sydney

WO2022045231A1 - Ester compound - Google Patents
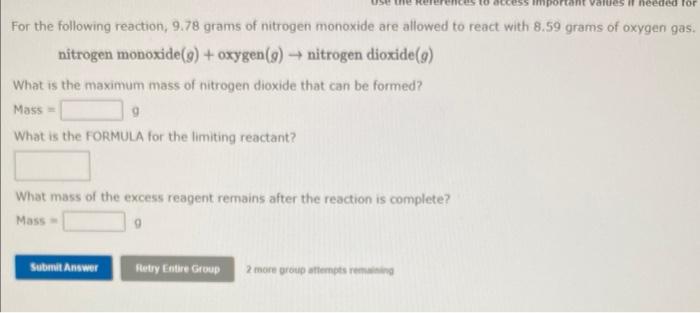
Solved For the following reaction, 9.78 grams of nitrogen

Empirical Formula from Combustion - Carbon, Hydrogen AND oxygen
You may also like






Related products



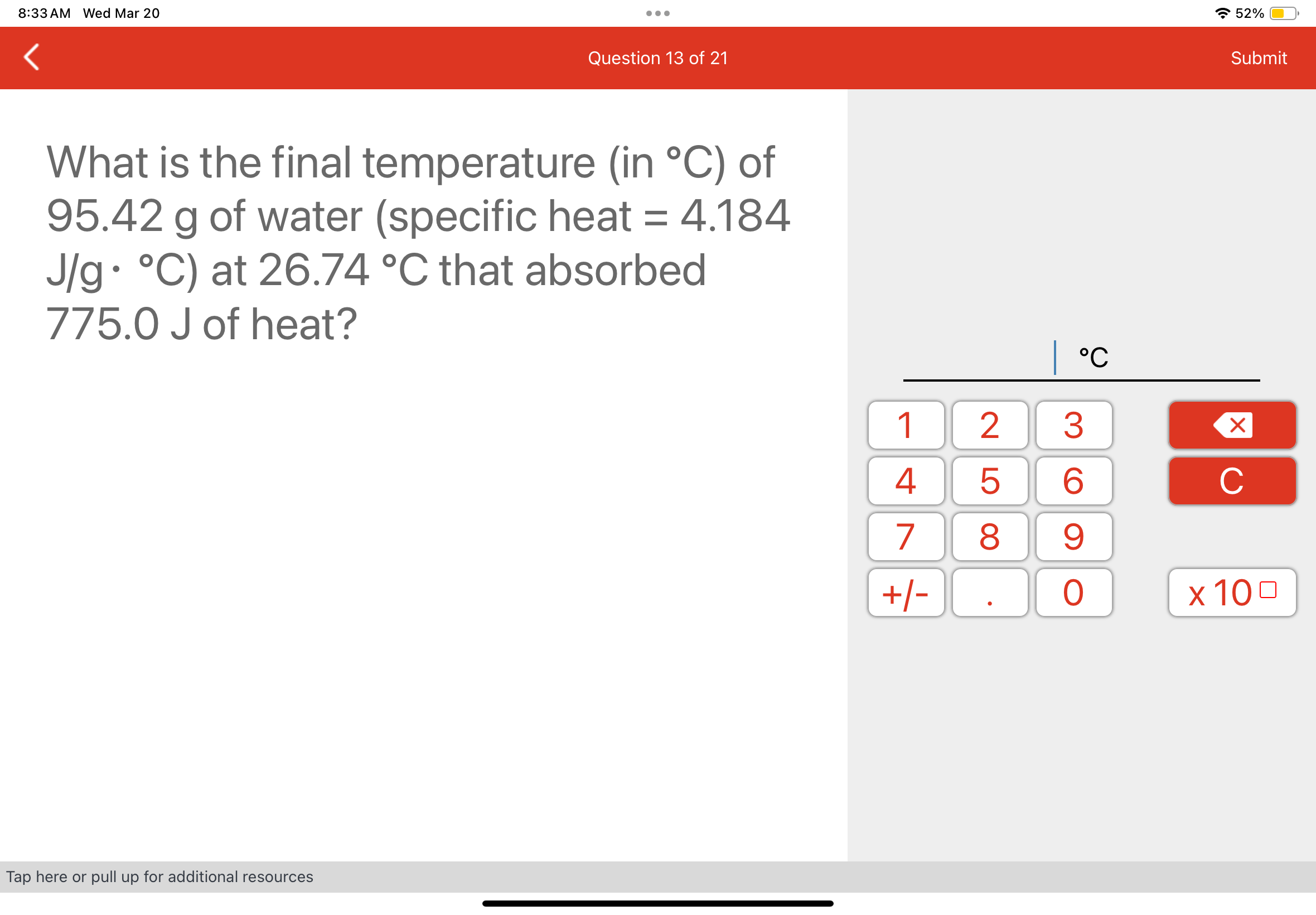

© 2018-2024, bellvei.cat, Inc. or its affiliates
