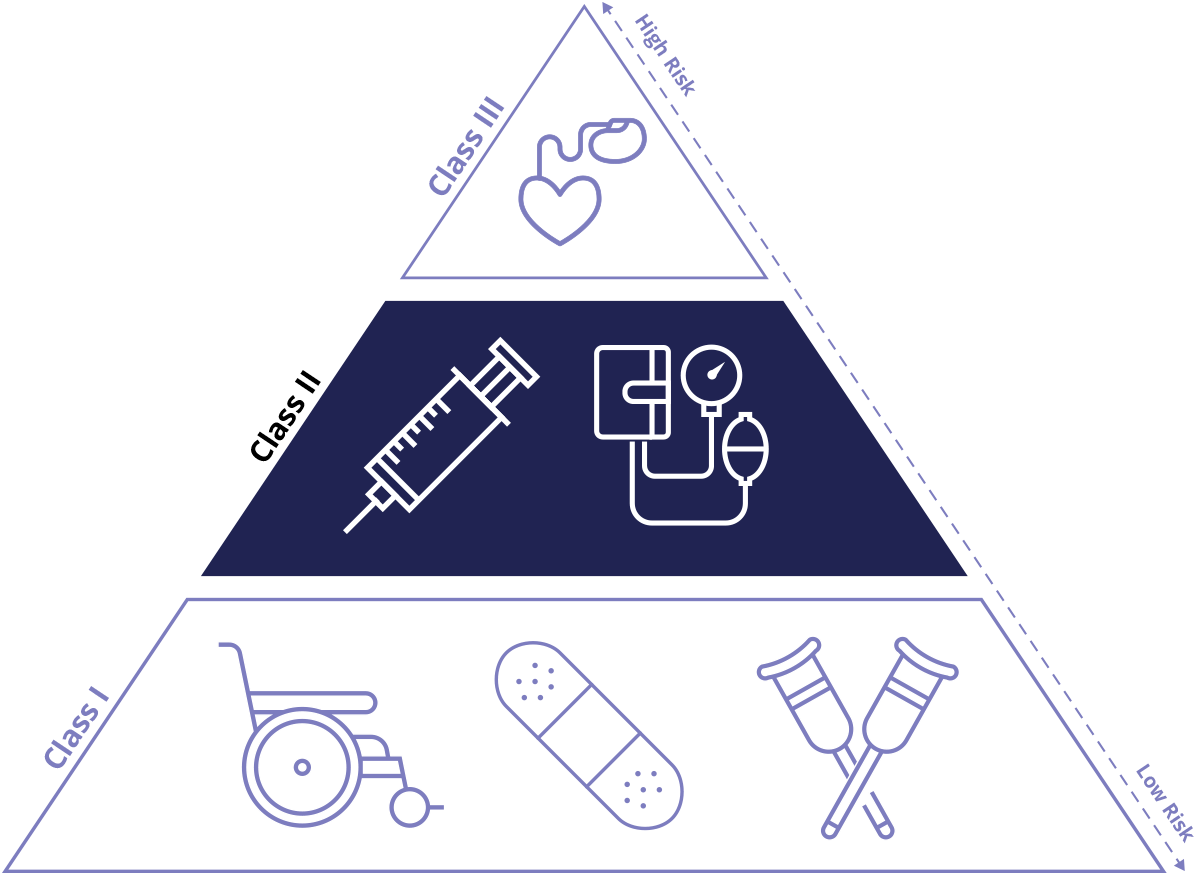
Class II medical devices have moderate to higher risks to patients or users. Over 40% of medical devices fall into this device category. The majority of medical devices are considered to be Class II devices. Some examples of Class II devices include catheters, syringes, contact lens, and pregnancy test kits.
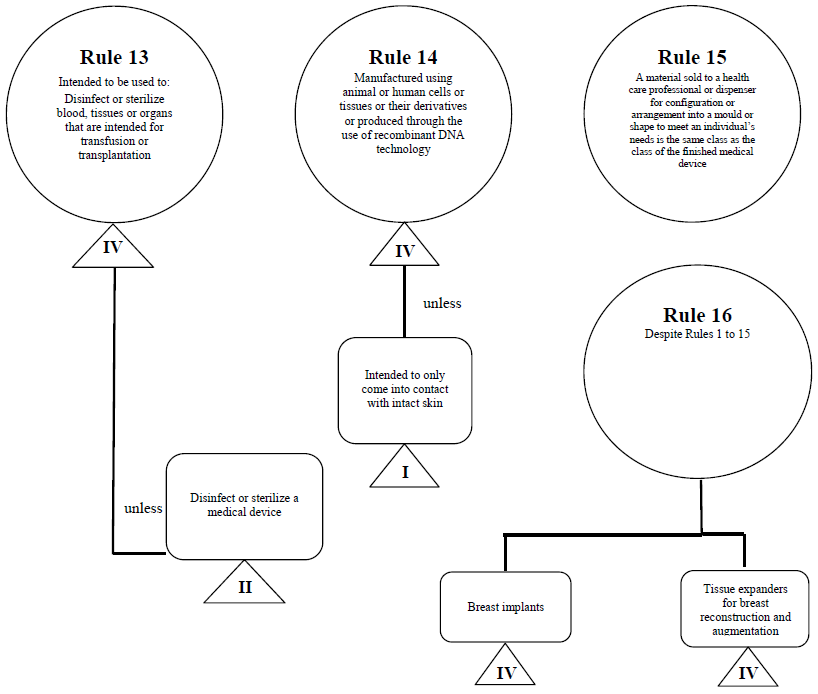
Guidance Document - Guidance on the Risk-based Classification System for Non-In Vitro Diagnostic Devices (non-IVDDs)
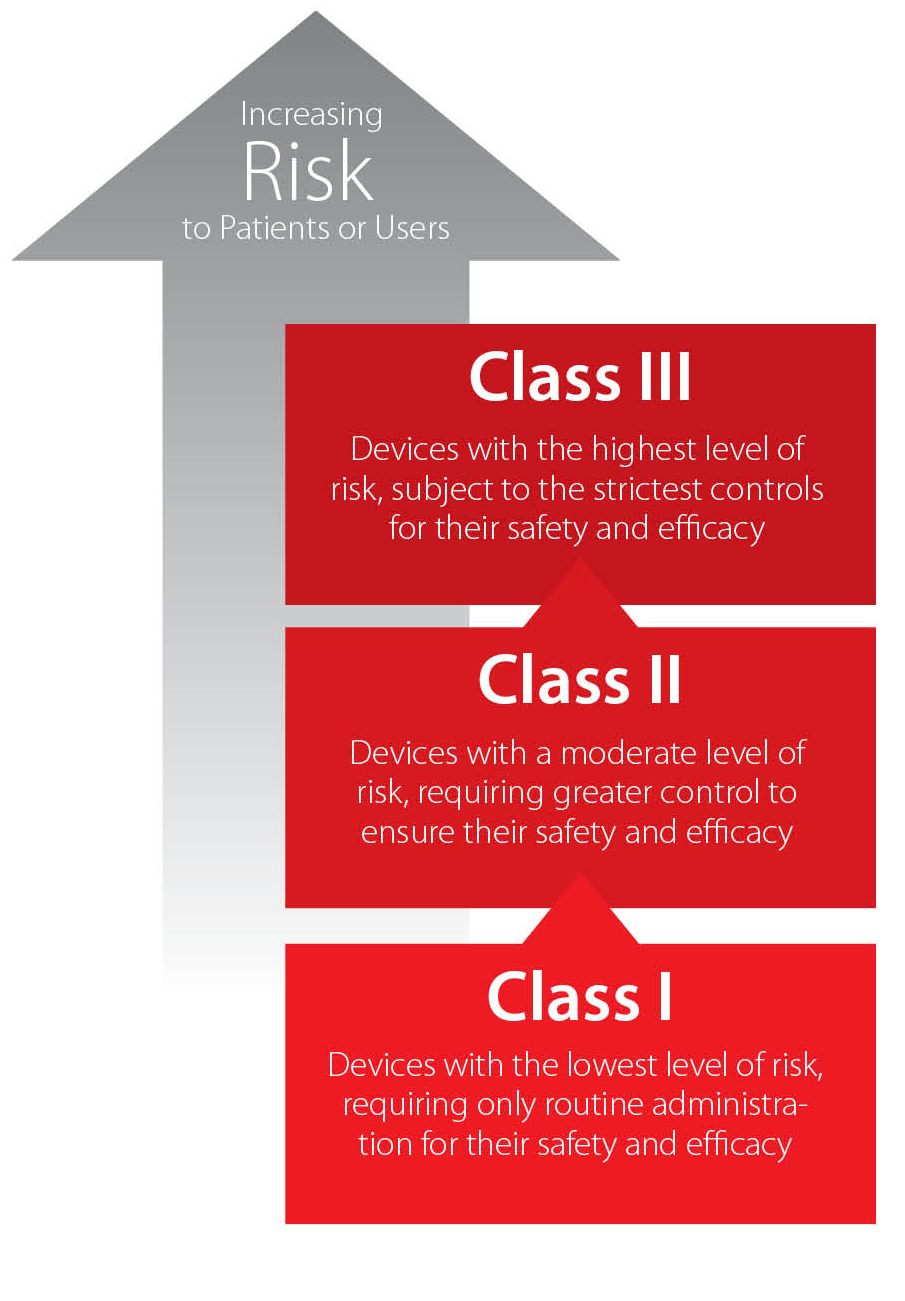
Investing in China's Medical Device Industry, Part 1 - China Briefing News

MDR - Classes and Conformity - tracekey solutions GmbH

EU MDR Compliance on LinkedIn: #medicaldevices #medicaldevice #risk #classifcation #us #fda #eumdr…
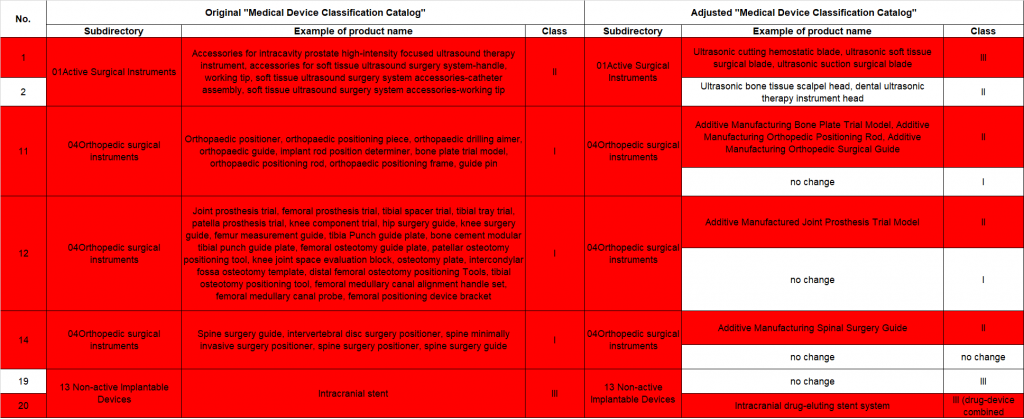
China medical device classification catalogue updates
What is a Class 2 Medical Device in the US?
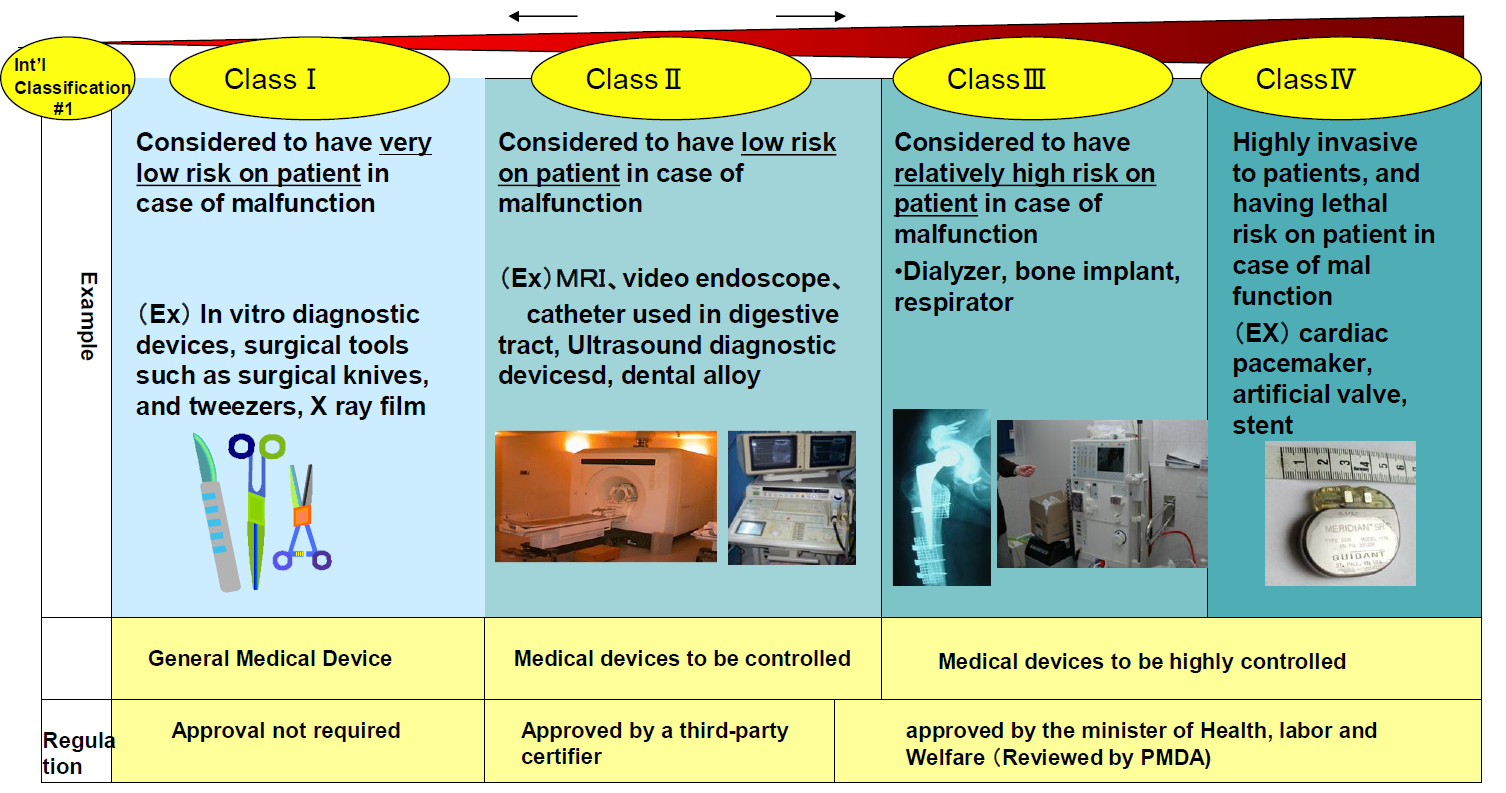
Interoperability standards for medical device integration in the OR and issues relating to international approval procedures (part 4) - ISCASBlog

Guidance Document - Guidance on the Risk-based Classification System for Non-In Vitro Diagnostic Devices (non-IVDDs)

Navigating Medical Device Regulations: A Comparative Analysis of EU MDR and US MDR, by KSHITIJ SHARMA
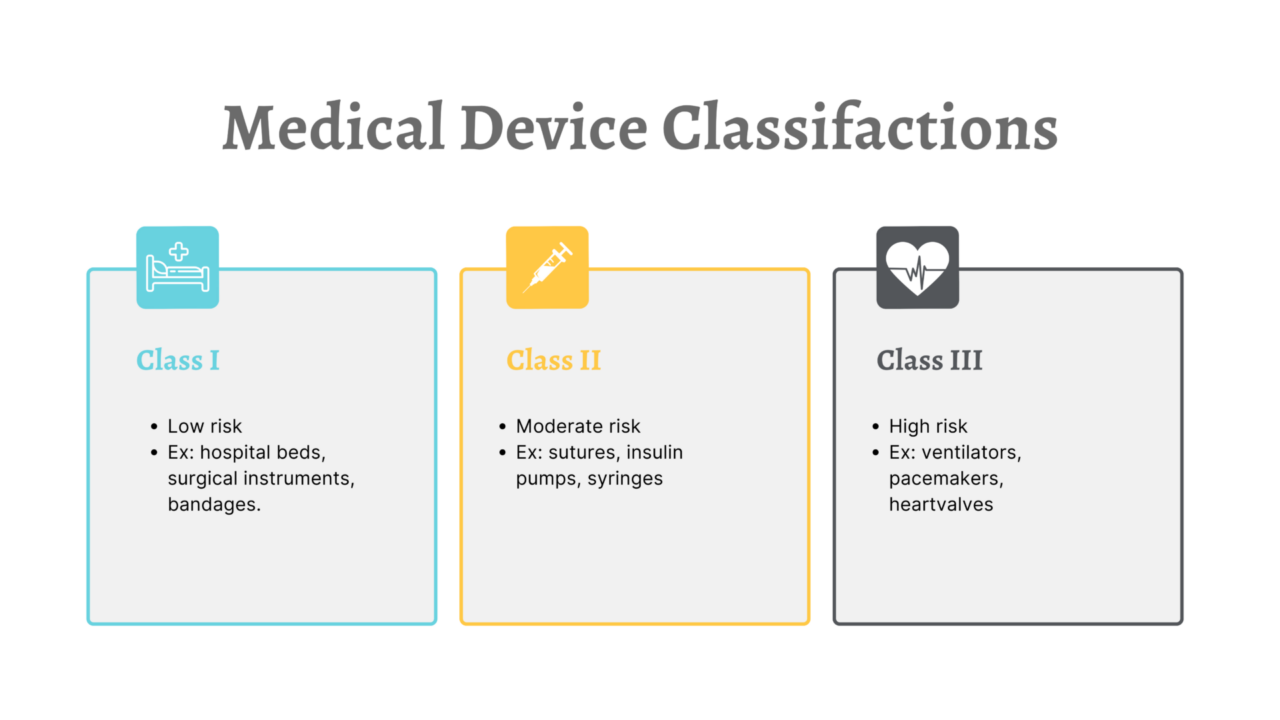
510(k) Exempt Medical Devices - Overview
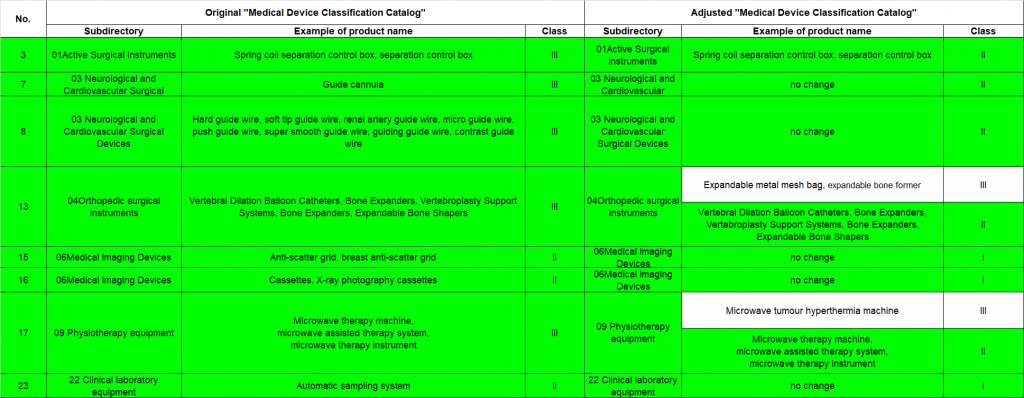
China medical device classification catalogue updates

New world order 2013

FDA Medical Device Classification. : PresentationEZE