FDA Enhances Global Patient and Regulatory Collaborations in Oncology
4.5 (533) · $ 9.99 · In stock
In recognition of World Cancer Day 2024, the FDA and European Medicines Agency will collaborate to spotlight innovative cancer treatment advances for patients.

Applying RWE to Regulatory Applications
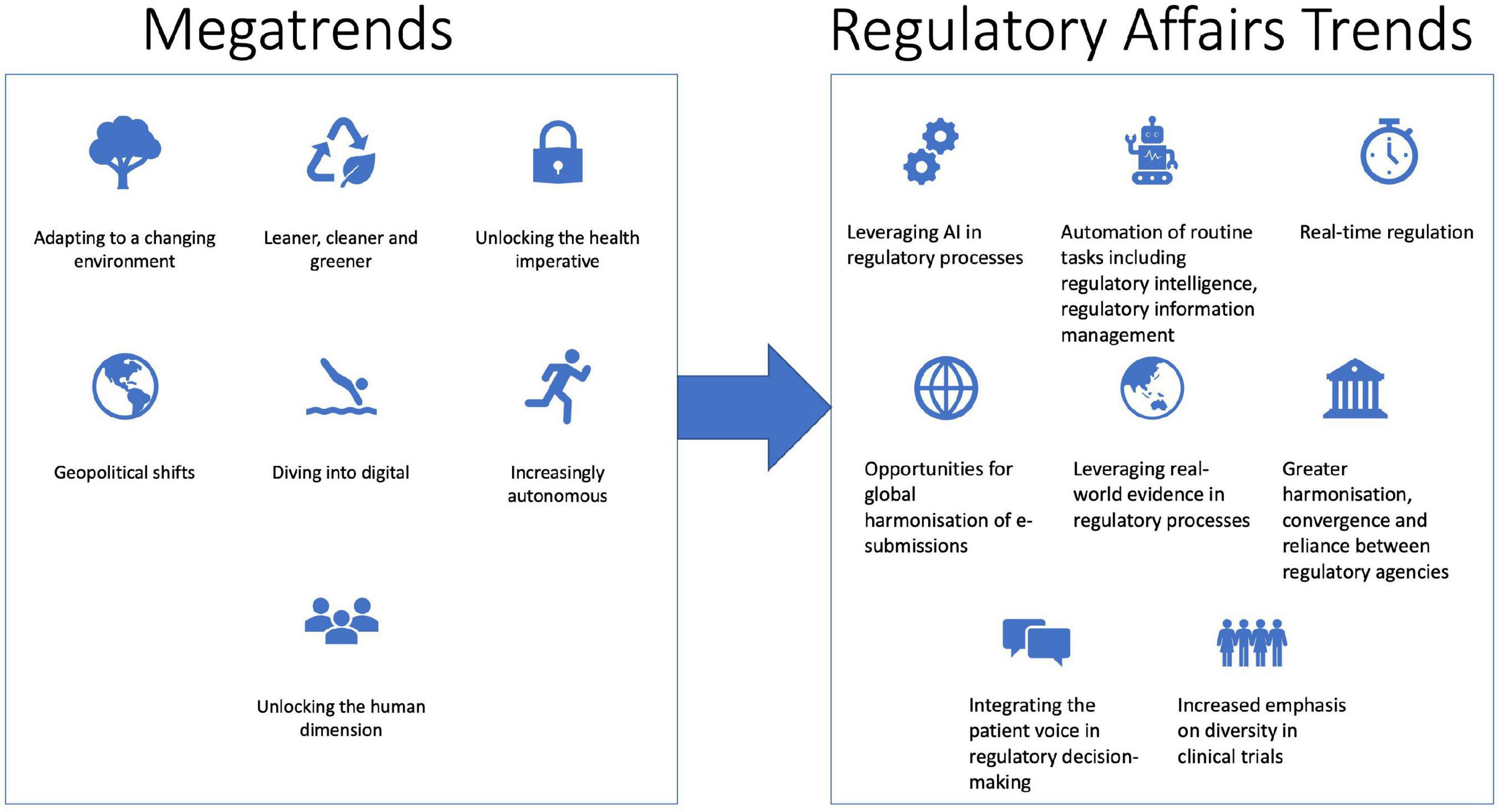
Frontiers Future directions in regulatory affairs

FDA Voices

victor cerasale on LinkedIn: The Lancet Digital Health Homepage

Convergence in the Global Regulatory Village

SCRI Announces a Collaboration with AstraZeneca Focused on Technology Enhancements & Innovative Operational Model to Advance Cancer Research

Project Community

Mazada Pharma Guide

FDA's Digital Leap: What are the Latest Digital Health Updates from the Agency? – Digital Medicine Society (DiMe)
You may also like






Related products





© 2018-2024, bellvei.cat, Inc. or its affiliates
