PDF] Hemoglobin polymorphism in white-tailed deer: subunit basis
4.9 (621) · $ 22.99 · In stock
It was concluded from the results of limited structural studies that there were multiple peptide differences upon comparison of three non-α polypeptide chains in white-tailed deer. A variety of aberrant erythrocyte forms have been related to seven adult and two fetal hemoglobins in white-tailed deer. While sickling of the erythrocyte was not associated with a single hemoglobin type, it was precluded by hemoglobin V or VII, even when in combination with other hemoglobin types normally associated with sickling. The subunit basis of the hemoglobin polymorphism was presented. Two kinds of α subunits, six kinds of β subunits and one γ subunit were related to the whole hemoglobin molecule. The heterogeneity of the deer hemoglobins was based upon a variety of combinations of these numerous polypeptide chains. It was concluded from the results of limited structural studies that there were multiple peptide differences upon comparison of three non-α polypeptide chains.

High-altitude deer mouse hypoxia-inducible factor-2α shows

PDF) Hemoglobin polymorphisms affect the oxygen binding properties

(PDF) Pan-American Trypanosoma (Megatrypanum) trinaperronei n

Relaxed functional constraints on triplicate α-globin gene in the

Ultrastructure of Sickled Deer Erythrocytes. I. The Typical
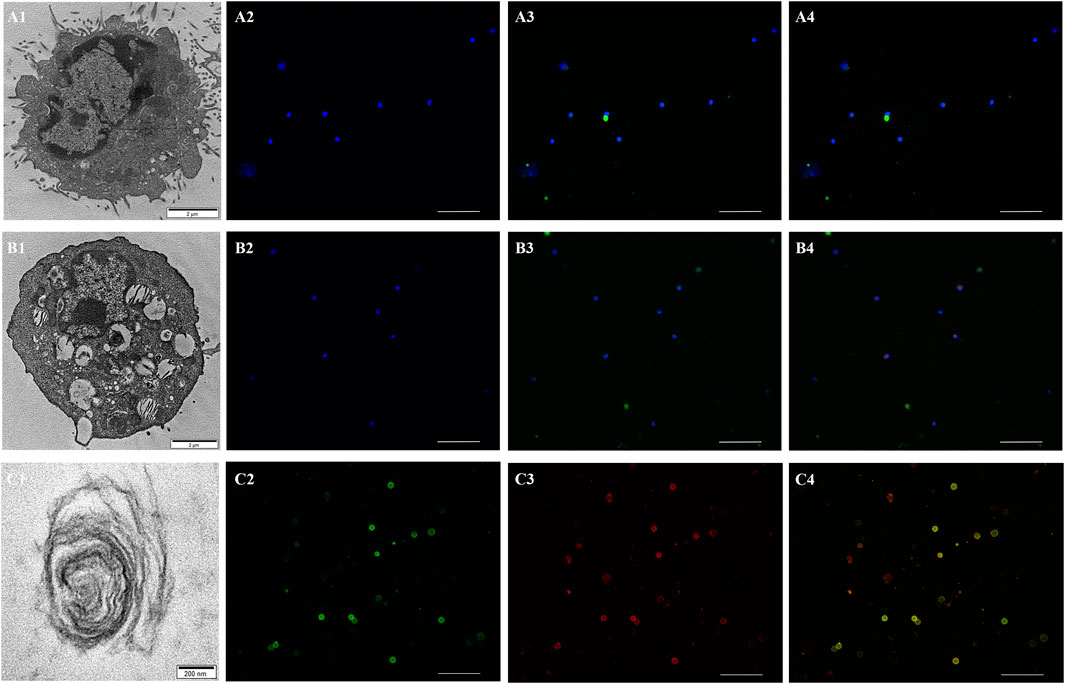
Frontiers A New Homotetramer Hemoglobin in the Pulmonary

PDF) Parallel evolution in the major hemoglobin genes of eight

Ultrastructure of Sickled Deer Erythrocytes. I. The Typical

A new era for understanding amyloid structures and disease

,aspect=fit)








![PDF] Hemoglobin polymorphism in white-tailed deer: subunit basis](https://d3i71xaburhd42.cloudfront.net/442de7651b90997b8b5c2ac0309ecaf101fed8da/4-Table1-1.png)