FDA Issues New Mammography Guidelines for Women With Dense Breasts
4.8 (360) · $ 5.99 · In stock
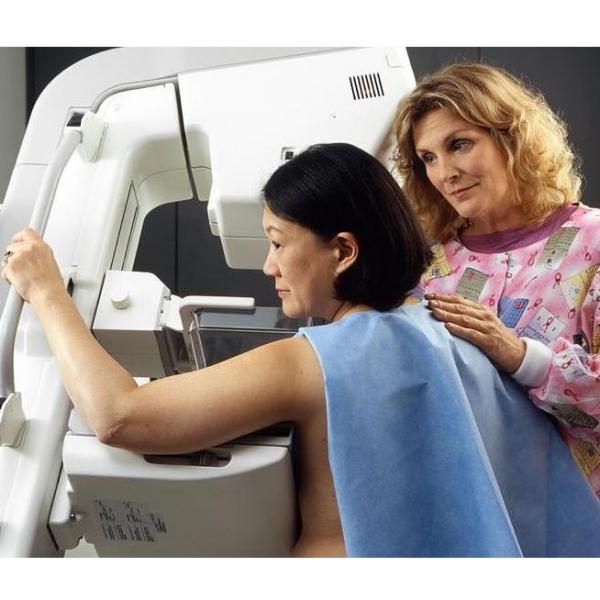
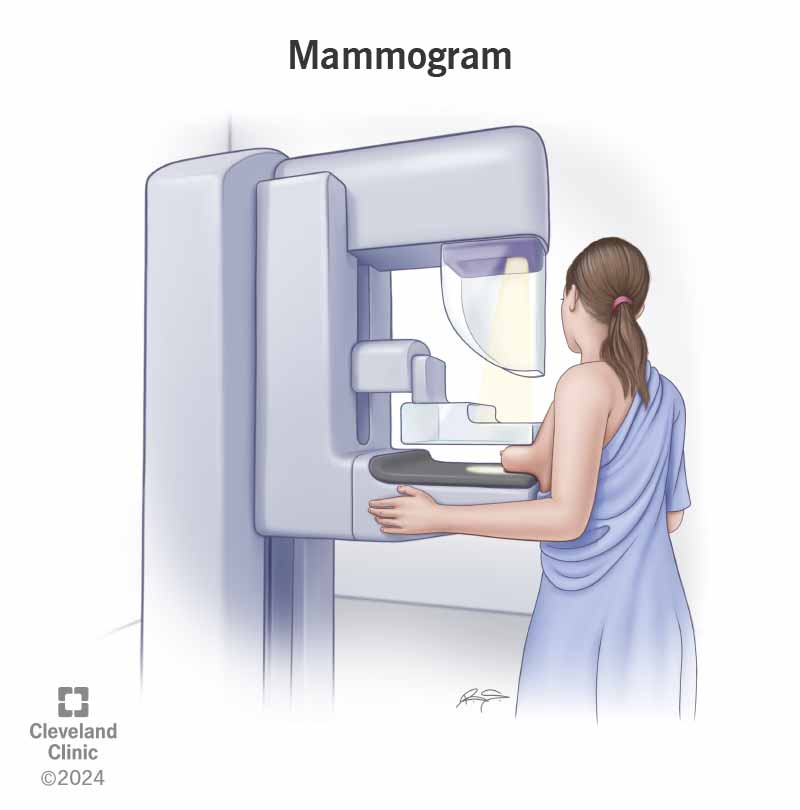
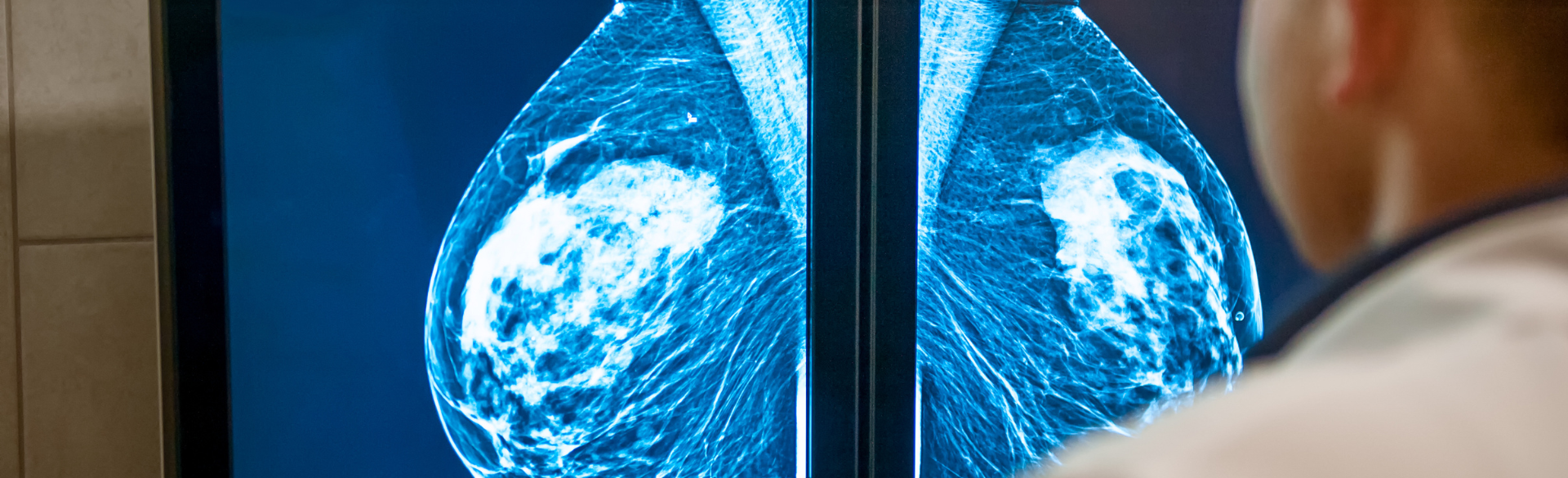
The FDA on March 9 updated its mammography guidelines to require mammography facilities to notify patients about the density of their breasts.
New FDA guidelines on breast density notifications and implications for overuse - Lown Institute
Mayo Clinic offers new guidance on supplemental screening of women with dense breasts

FDA Will Require Dense Breast Disclosure at Mammogram Clinics - The New York Times

The FDA Proposed New Mammogram Guidelines That Would Make Detecting Breast Cancer Early Easier

Are Your Breasts DENSE or NOT DENSE? - Elizabeth Wende Breast Care

Mammography vs. thermography: Making an informed decision
FDA issues new mammogram regulations aimed at further breast cancer prevention

CU Cancer Center
CU Department of Surgery Top Stories of 2023

Medical practitioners will have to notify patients about breast density in mammograms under new FDA regulations - CBS News