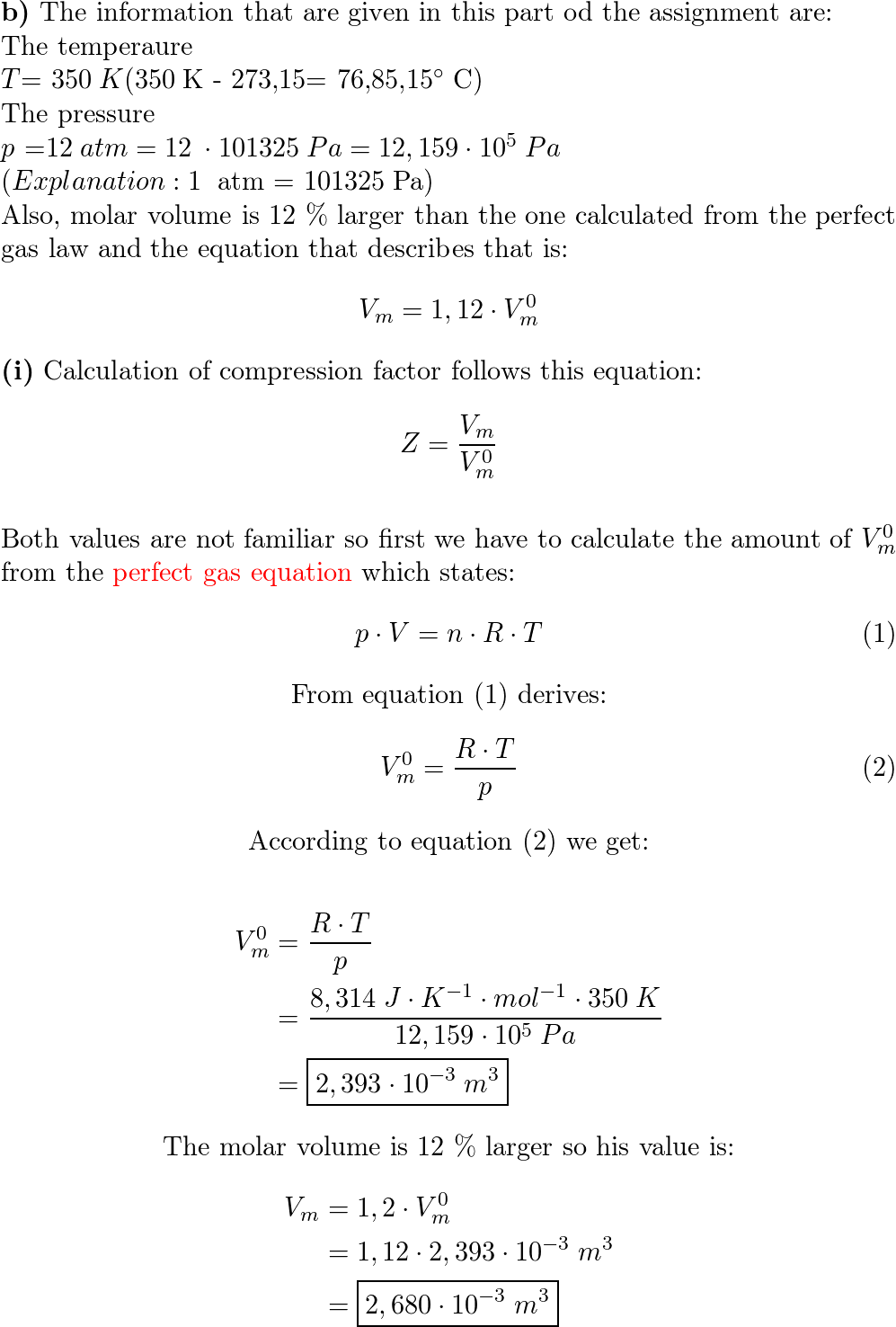
Developing a Thermodynamical Method for Prediction of Activity
4.9 (282) · $ 14.50 · In stock
Results of the experimental measurements on the partial molar volume of kerosene used as a medium for dissolving TBP are utilized to determine the activity of TBP in the binary kerosene-TBP solution through the application of Gibbs-Duhem equation. The treatment is based on combination of the experimental data with the thermodynamic values available on the compressibility factor of pure kerosene at room temperature. It is shown that the activity of TBP in kerosene has a positive deviation from ideality with an activity coefficient derived as follows:1) at X TBP ≤ 0.01: γ TBP = 42.530, 2) at the 0.01 X TBP 0.2: 3) at the higher TBP concentrations 0.2 X TBP 0.97: and 4) at TBP Raoultian concentrations 0.97 ≤ X TBP:γ TBP = 1. These quantities can be utilized at temperature closed to 298 K.

Water adsorption in the organic phase for the D2EHPA-kerosene/water and aqueous Zn2+, CO2+, Ni2+ sulphate systems

A comparison between TEHA and Cyanex 923 on the separation and the recovery of sulfuric acid from aqueous solutions

Molecular thermodynamics for scaling prediction: Case of membrane distillation - ScienceDirect

DeepTM: A deep learning algorithm for prediction of melting temperature of thermophilic proteins directly from sequences - Computational and Structural Biotechnology Journal

Thermodynamic Properties of a Solution of 2-Ethoxyethanol in Jet Kerosene
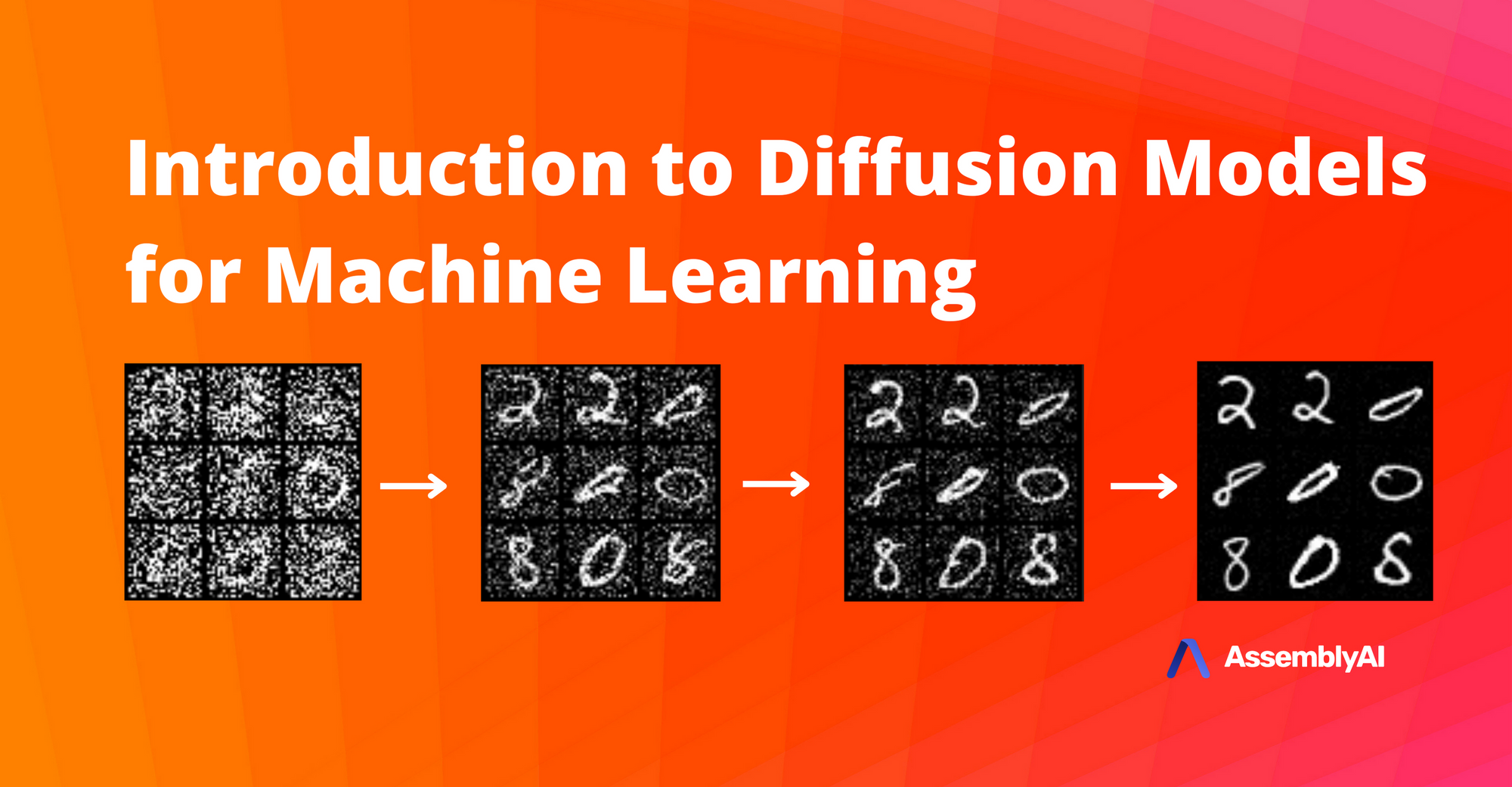
Introduction to Diffusion Models for Machine Learning
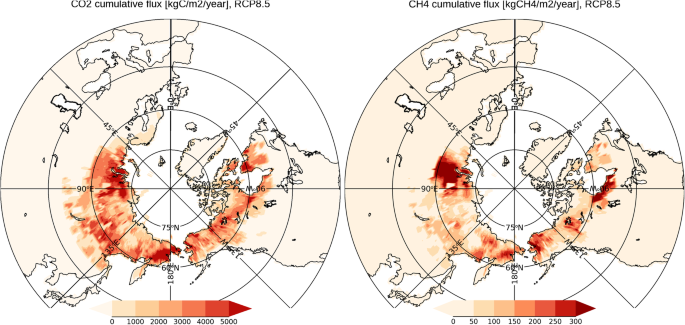
Future projection of greenhouse gas emissions due to permafrost degradation using a simple numerical scheme with a global land surface model, Progress in Earth and Planetary Science
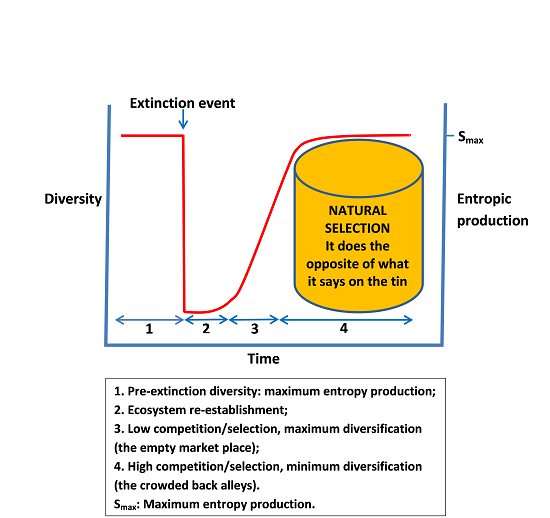
Entropy, Free Full-Text

PDF) Developing a Thermodynamical Method for Prediction of Activity Coefficient of TBP Dissolved in Kerosene

E. ALAMDARI, Professor (Associate), PhD, Amirkabir University of Technology, Tehran, TUS, Department of Mining and Metallurgical Engineering

PDF) Thermodynamics of extraction of Zn2+ from sulfuric acid media with a mixture of DEHPA and MEHPA

Synergistic effect of MEHPA on co-extraction of zinc and cadmium with DEHPA

Thermodynamic modelling - Wikipedia

Thermodynamics and Kinetics Group

Analyzing Some Elements of Technological Singularity Using Regression Methods

)