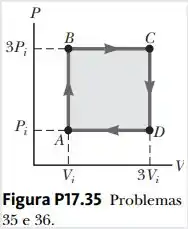
Solved An ideal gas initially at Pi, Vi, and Ti is taken
4.5 (325) · $ 10.99 · In stock

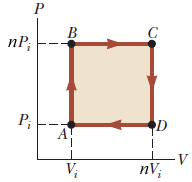
An ideal gas initially P_i ,V_i , and T_i is taken through a cycle as shown in Figure. (a) Find the net work done on the gas per cycle 1.00 mol of

SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown in the figure below where n = 2. (Use any variable or symbol stated above
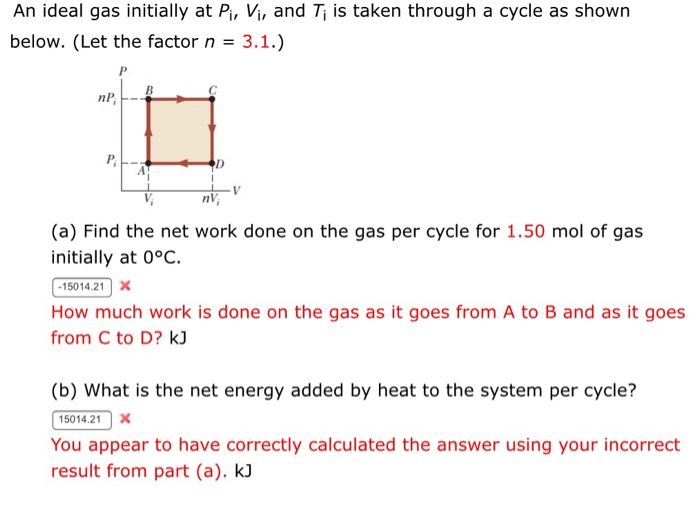
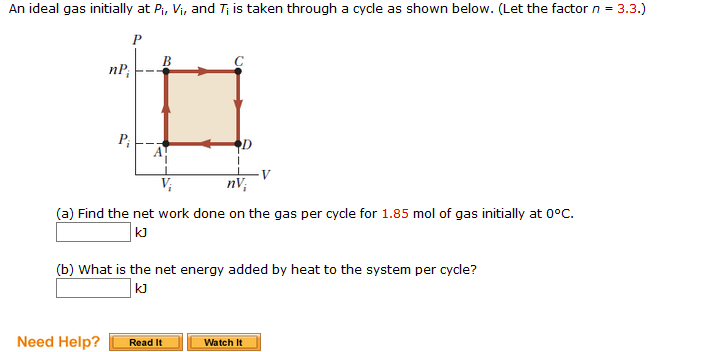
Solved An ideal gas initially at Pi, Vi, and Ti is taken

SOLVED: Diatomic ideal gas (γ = 1.40) confined to a cylinder through a closed cycle. Initially, the gas is at Pi, Vir, and Ti. First, its pressure doubles under constant volume. It
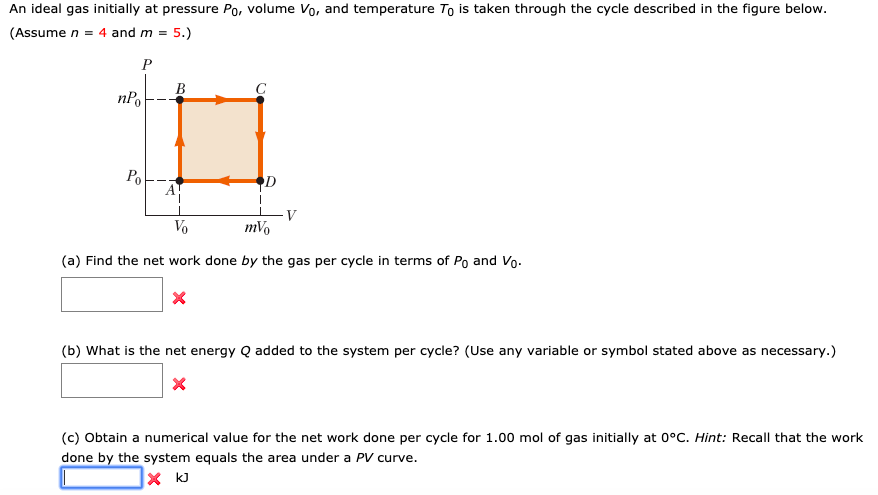
Solved An ideal gas initially at pressure P0, volume V0, and

A Quantum Theory for Bose–Einstein Condensation of the Ideal Gas – Quantum
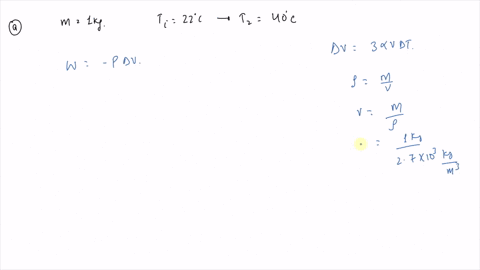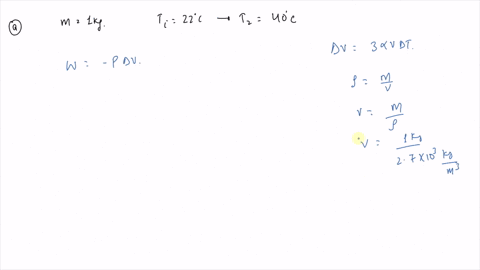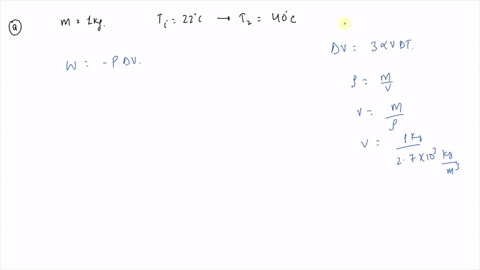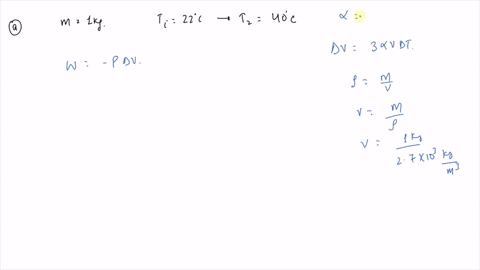
⏩SOLVED:An ideal gas initially at Pi, Vi, and Ti is taken through a…

SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown in the figure (Pressure in Pa and Volume in m^3) What is the net energy
Solved An ideal gas initially at Pi, Vi, and Ti is taken

Thermodynamics problems