The compressibility factor Z for an ideal gas will be
4.5 (488) · $ 25.99 · In stock
The compressibility factor Z for an ideal gas will be
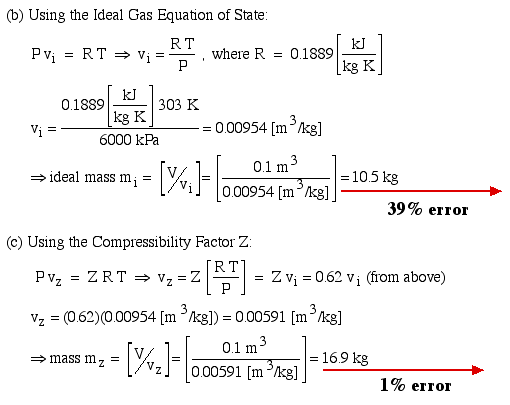
Chapter 2b: Pure Substances: Ideal Gas (updated 1/17/11)

Question No 5 4 Digit Integer Type Question Q.1 to Q.6 are

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics
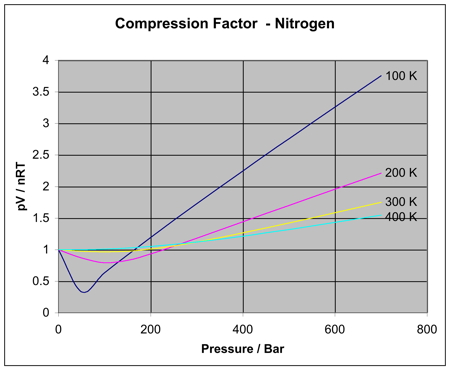
Non-ideal behavior of gases (article)

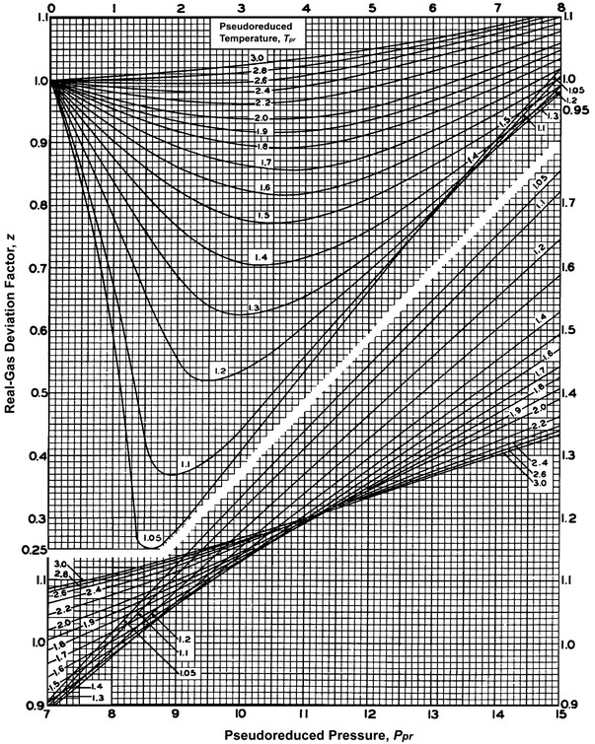
Plot of experimental measurements of the z-factor
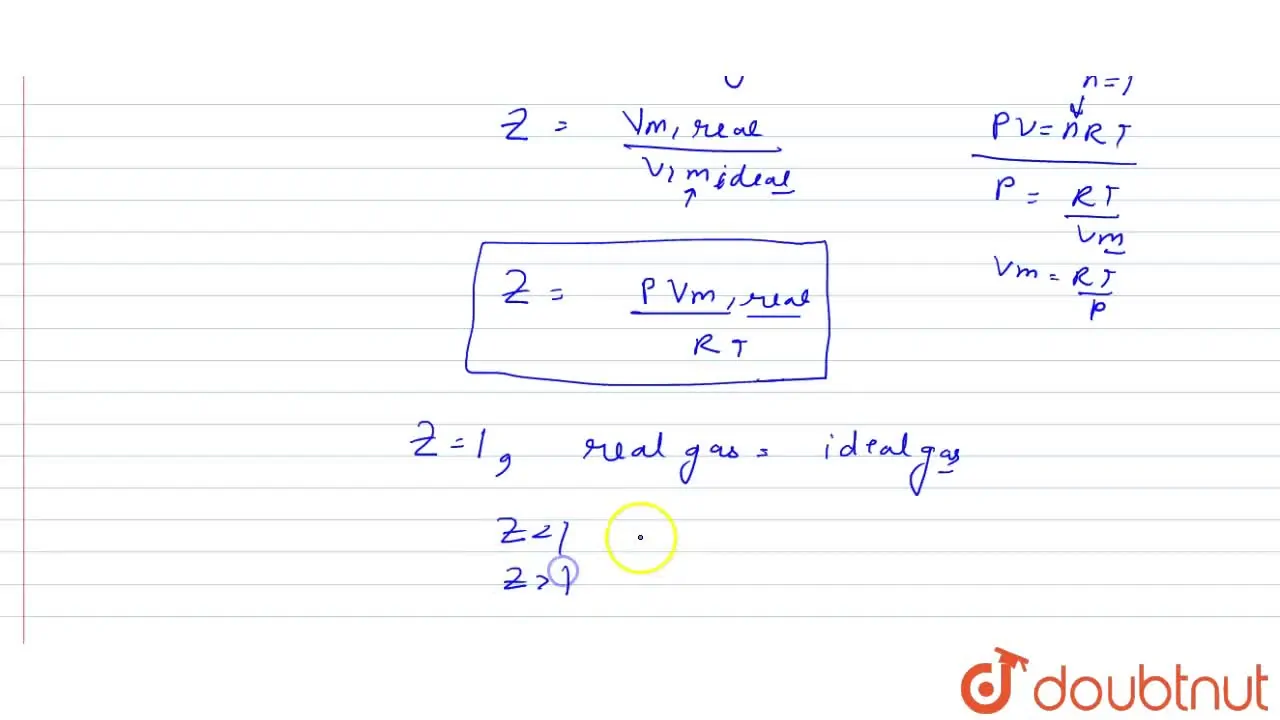
How is compressibility factor expressed in terms of molar volume of th

Compressibility factor (gases) - Citizendium

Z lt 1 and attractive forces are dominant

The compressibility factor Z = ((PV)/(nRT)) of a gas above T = (a)/(Rb
For a non-ideal gas, the compressibility factor (Z) is defined as: Z

For a non-ideal gas, the compressibility factor (Z) is defined as: Z

Real Gas Behavior The Compression Factor (Z) [Example #2]

36. question is a single digit integer ranging from 0 to 9, both

Marathi] The compressibility factor of a gas is defined as z = PV //R