- Home
- 32 g
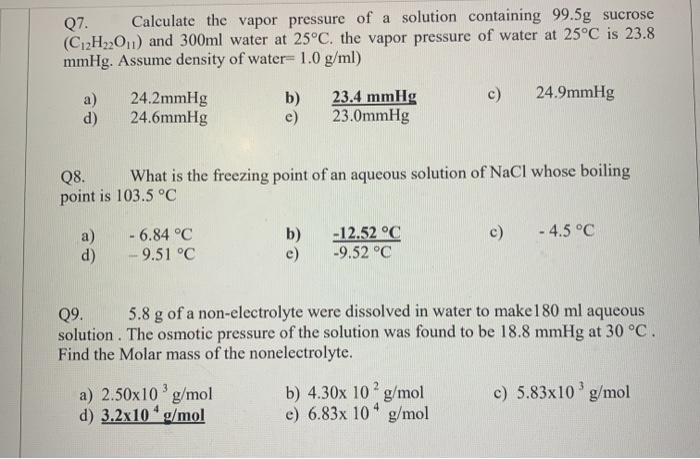
- The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1)
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1)
5 (105) · $ 16.50 · In stock
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1) X 2)X2 3)X4 4)X8
The vapour pressure of a solution having 2-0 g of solute X -gram atomic mass-32 g-mol- in 100 g of CS2 -vapour pressure -854torr- is 848-9 torr-The molecular formula of solute 1- X 2-X2 3-X4 4-X8

Solutions (Colligative Properties, Abnormality in Molar Mass) The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol -1) in 100 g of CS, (vapour

The vapour pressure of CS(2) at 50^(@)C is 854 torr and a solution o
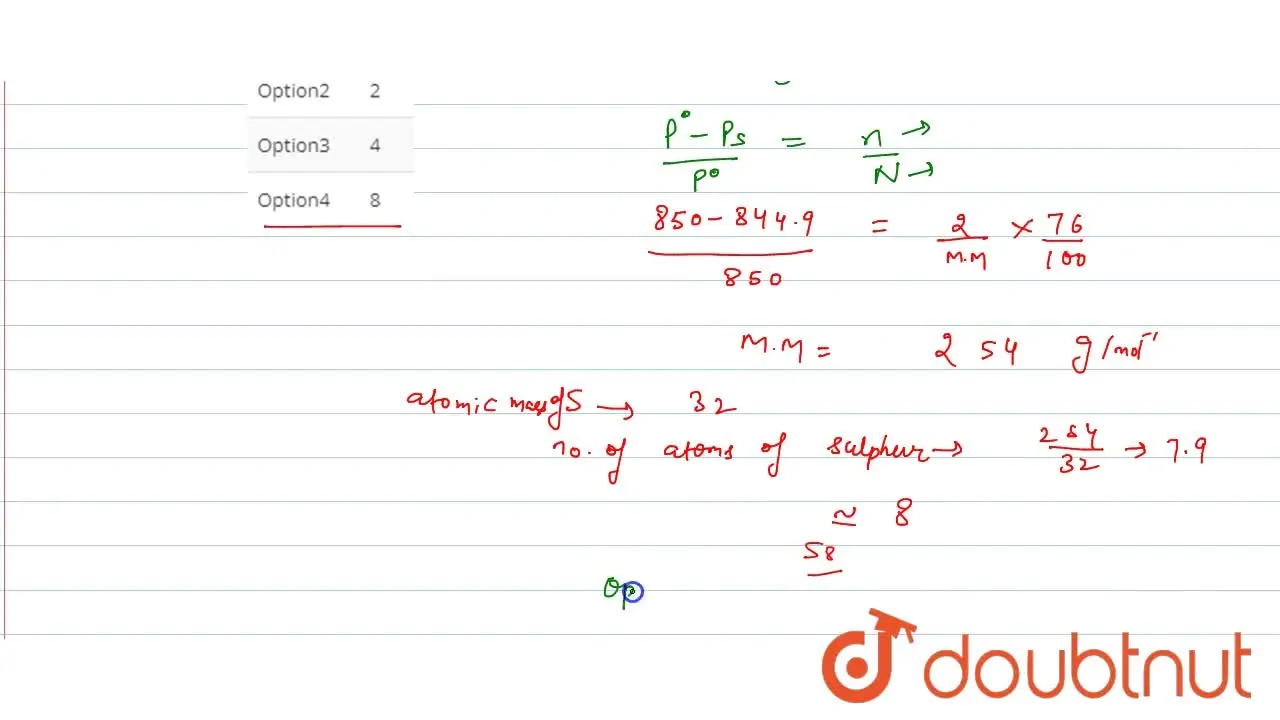
At 48^(@)C, the vapour pressure of pure CS(2) is 850torr . A solution
Stoichiometry Practice 2 answer key
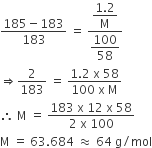
The vapour pressure of acetone at 20oC is 185 torr. When 1.2 g o


fraction olligative Properties, Abnormality in Molar Mass) uids has a boi..

Solution.pdf - Chemistry - Notes - Teachmint
Solved Q1. Complete the followings: (0.5 Mark each) a) If a

vapour pressure of solution of urea is 736.2 mm at 100 degress celsius calculate osmotic pressure of this solution at 15 degress celsius. - yvek0hss
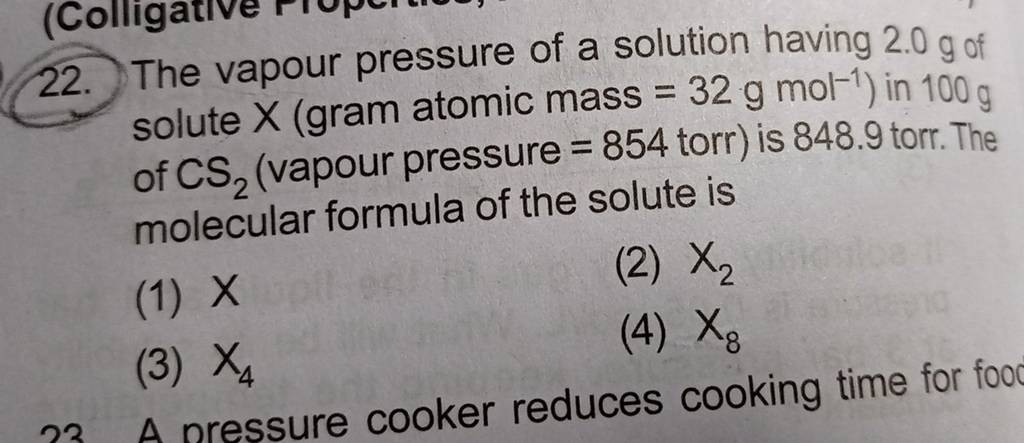
The vapour pressure of a solution having 2.0 g of a solute X( molar mass ..
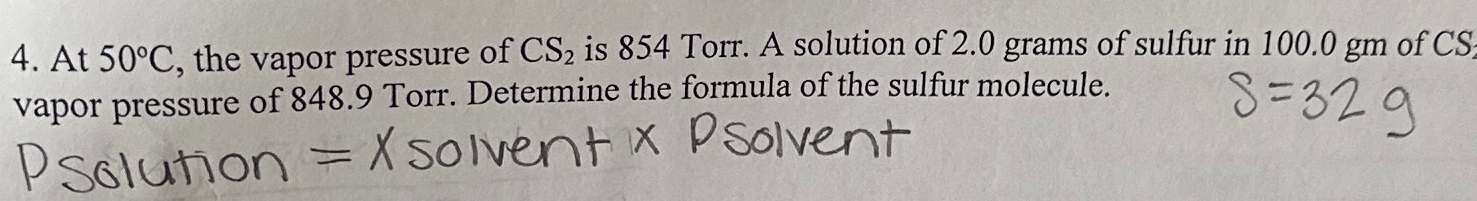
Solved At 50°C, the vapor pressure of CS2 is 854 Torr. A