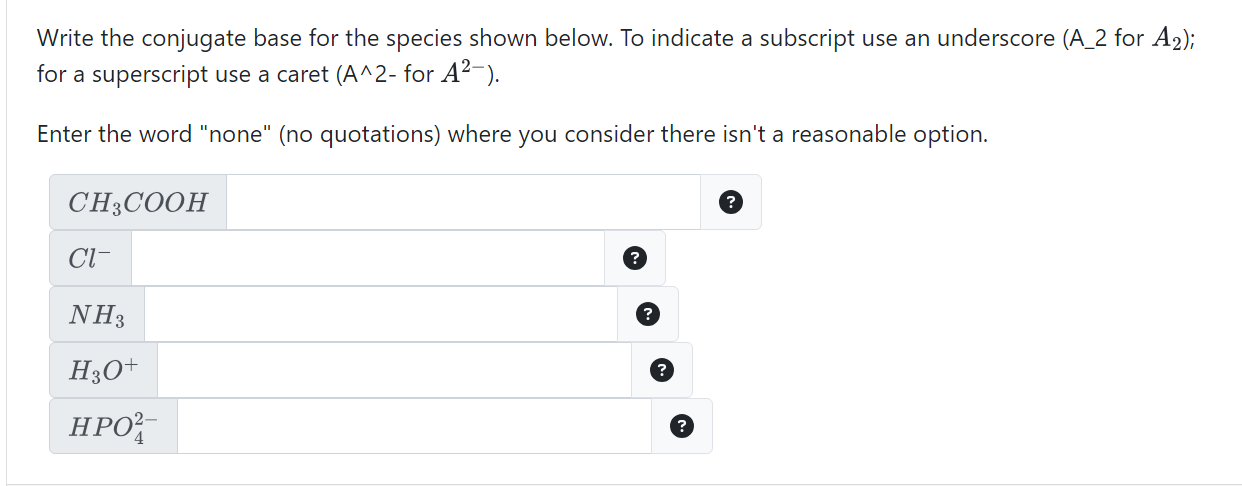
Solved Write the conjugate base for the species shown below
4.8 (235) · $ 15.50 · In stock
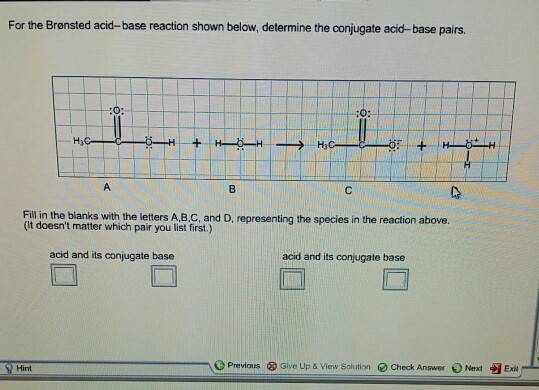
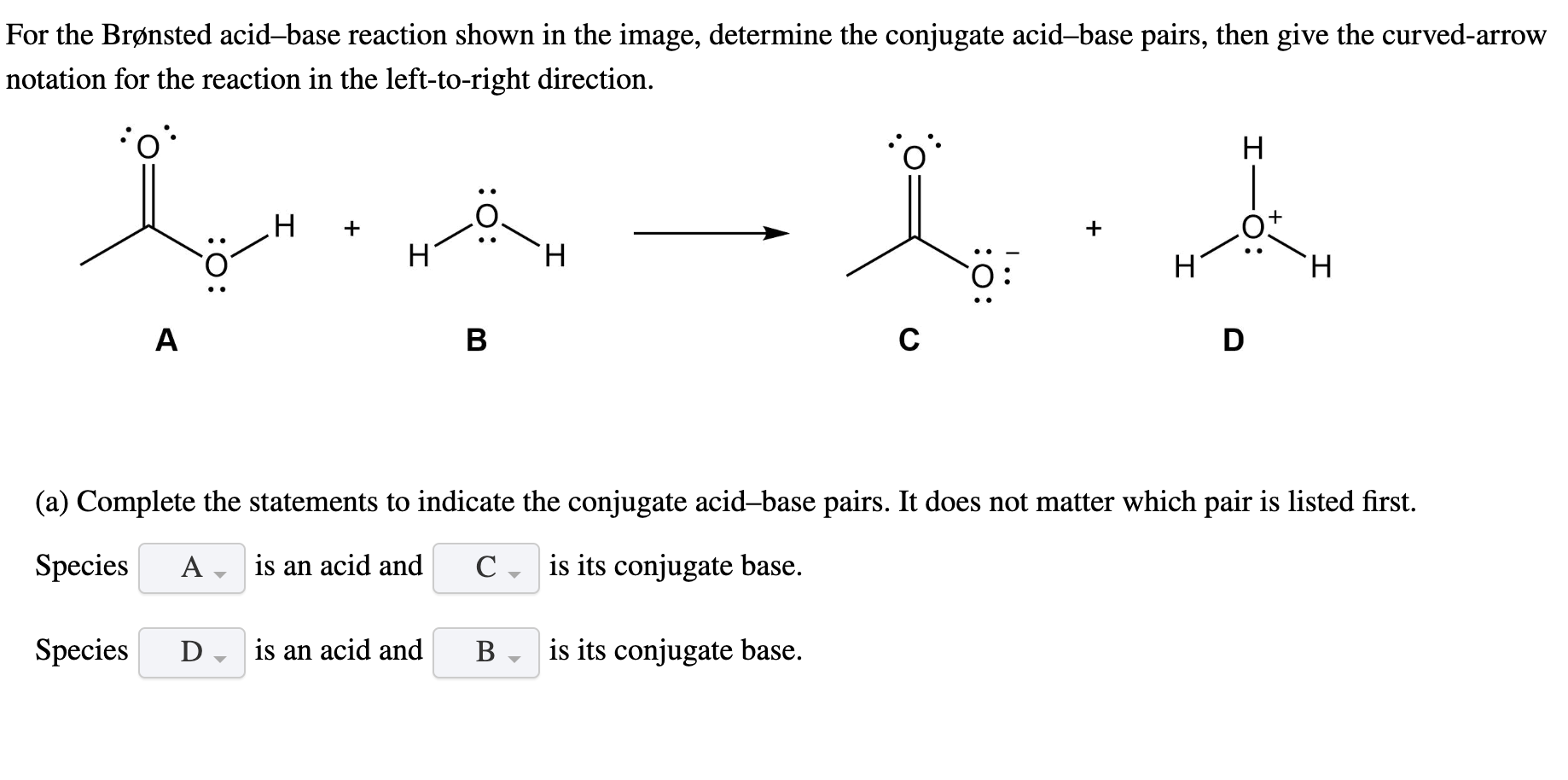
Solved For the Bronsted acid-base reaction shown below

SOLVED: Write the conjugate base for the species shown below. To indicate a subscript use an underscore (A2 for A2) for a superscript use a caret (A^2- for A2-) Enter the word
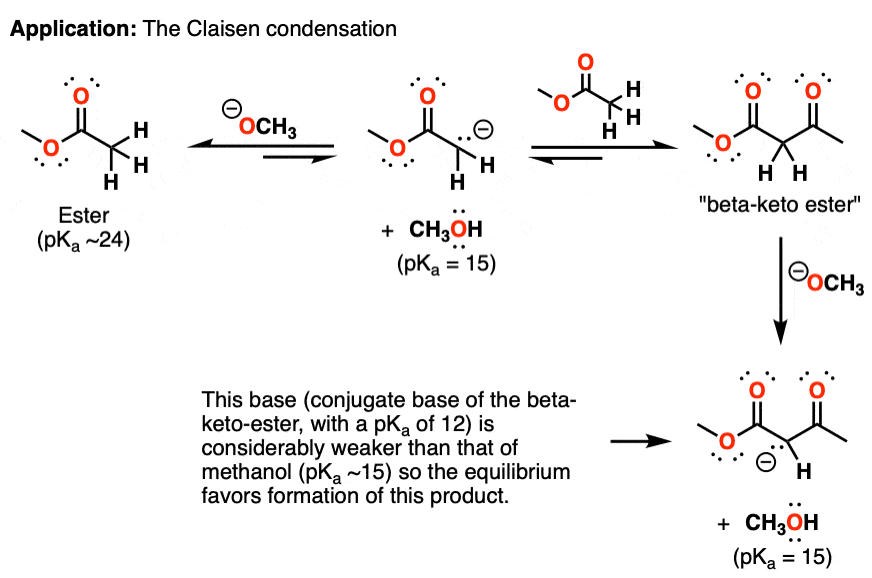
Reversible and Irreversible Acid-Base Reactions In Organic Chemistry

For each conjugate acid-base pair, identify the first species as an acid or a base and the second species as its conjugate acid or base. In addition, draw Lewis structures for each

Which statements best describe the relationship between phenol and phenoxide? More than one answer may be correct. a. Phenol is an acid and phenoxide is a base. b. Phenoxide is the conjugate
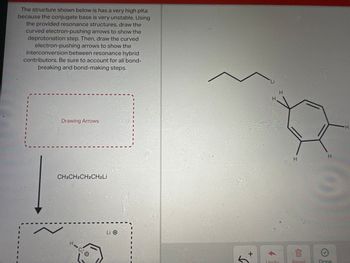
Answered: The structure shown below is has a very…

Malayalam] write the conjugate acid and base of the following species

For the following reaction, identify the reactant that is an acid, the reactant that is a base, and the two conjugate acid-base pairs present. OH-(aq) + HNO2(aq) arrow H2O(l) + NO2-(aq)

Ammonia appears in [TABLE 2-2 ] as both an acid and a conjugate b

How to Determine the Position of Equilibrium for an Acid–Base Reaction - Chemistry Steps
Solved it says the arrows i drew are incorrect. please
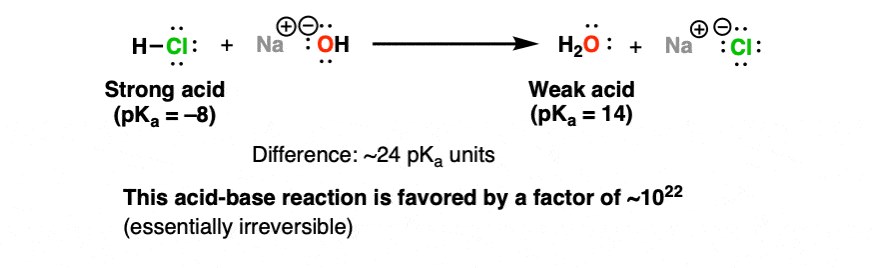
Reversible and Irreversible Acid-Base Reactions In Organic Chemistry
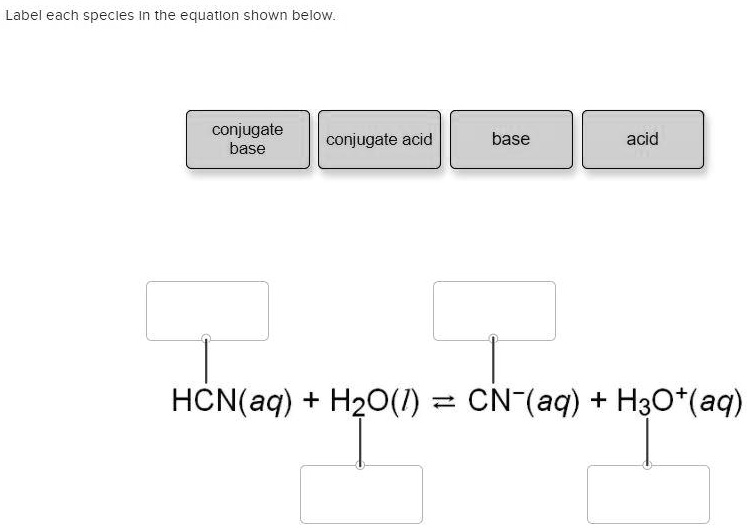
SOLVED: Label each species in the equation shown below: conjugate base conjugate acid base acid HCN(aq) + H2O(l) = CN-(aq) + H3O+(aq)