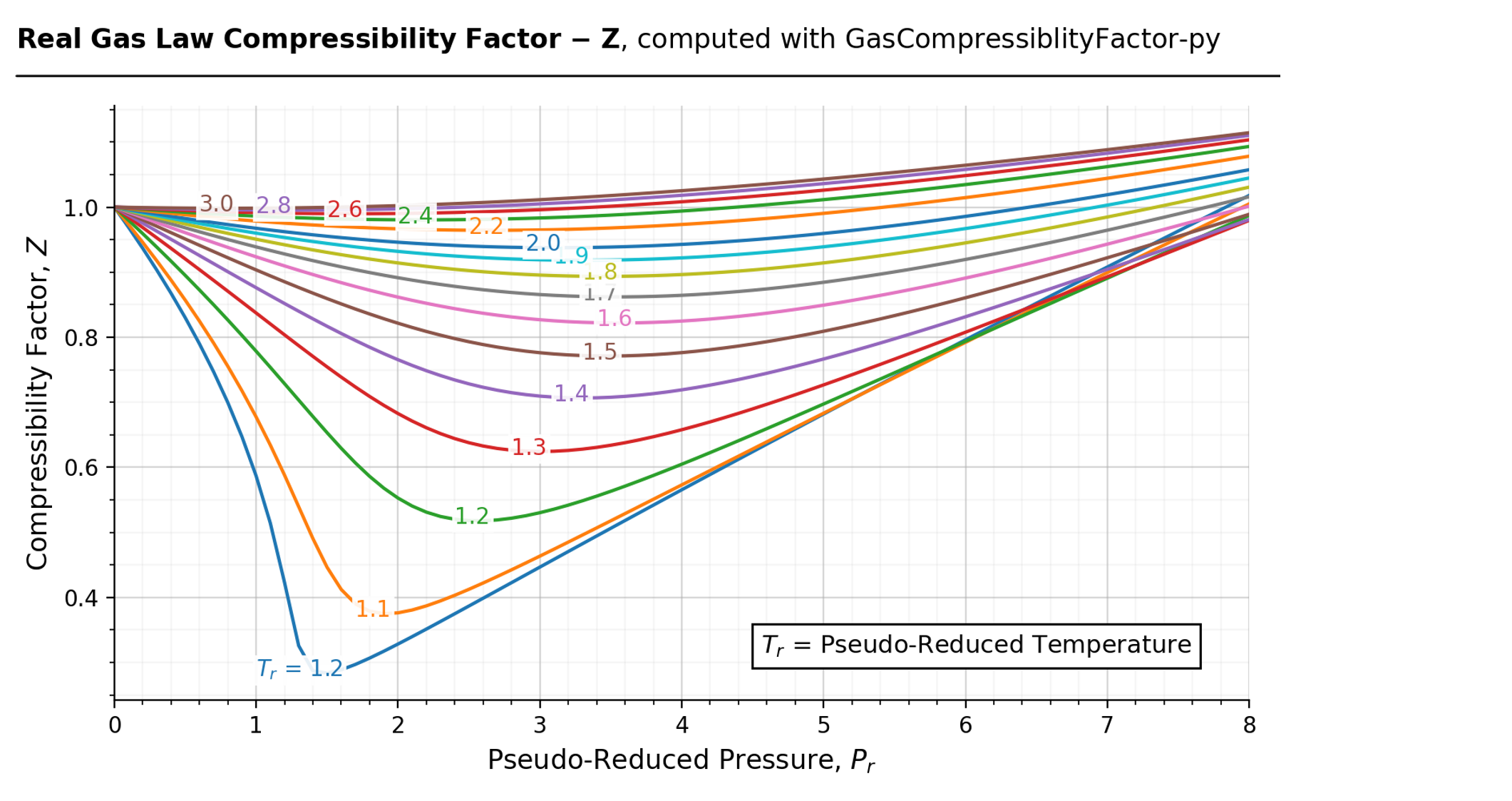
Solved The compressibility factor, Z, can be thought of as a
5 (520) · $ 17.99 · In stock
Answer to Solved The compressibility factor, Z, can be thought of as a

Solved Calculate and plot the compressibility factor Z for
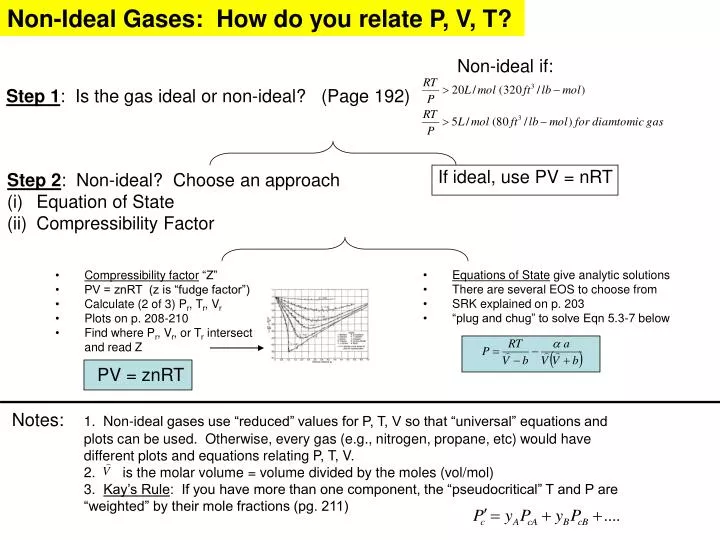
PPT - Step 1 : Is the gas ideal or non-ideal? (Page 192) PowerPoint Presentation - ID:3100094
Deviation of Gas from Ideal Behaviour and Its Causes

Gas Compressibility - an overview

The compressibility factor (Z) of one mole of a van der Waals' gas of negligible 'a ' value is:1dfrac{bp}{RT}1+dfrac{bp}{RT}1-dfrac{bp}{RT}

Real gases

The compressibility factor of a gas is defined as Z=P V / R T. The compressibility factor of idea

SOLVED: Derive the mathematical expression expressing the compressibility factor Z of a real gas depending on the reduced variables; Explain in detail how the volume of the actual gas at a given

Equation of state (excess compressibility factor Z À1 ¼ PV/(NkT) À 1 as

gas laws - Graph of compressibility factor vs pressure when real gas is assigned Z=1 - Chemistry Stack Exchange