What is the compressibility factor (Z) for 0.02 mole of a van der Waal
4.9 (187) · $ 16.50 · In stock
(d) (0.1+(1000xx(0.02)^(2))/(V^(2)))V=20xx0.02 =0.1V^(2)-0.4V+0.4=0 =V^(2)-4V+4=0 implies" "V=2L Z=(PV)/(nRT)=(0.1xx2)/(20xx0.02)=0.5

atm. 6. What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of lam. Assume the size of gas molecules is negligible. Given : RT = 20

⏩SOLVED:What is the compressibility factor (Z) for 0.02 mole of a
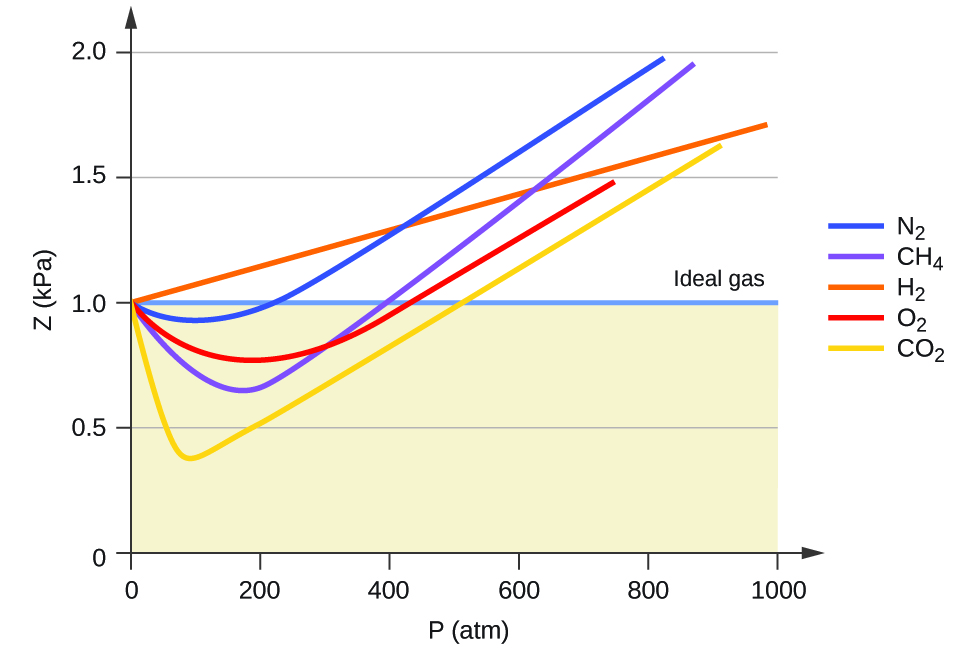
2.7: Non-Ideal Gas Behavior - Chemistry LibreTexts

Influence of Aprotic Cosolvents on the Thermophysical Properties

ecreases (C) remains same (D) changes unpredictably 16.a What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of 0.1 atm. Assume the size of gas molecules

0.585%NaCl solution at 27∘C has osmotic pressure of
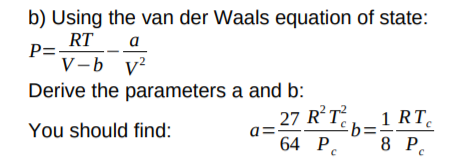
Answered: b) Using the van der Waals equation of…

Energies, Free Full-Text

SOLVED: A certain gas obeys the van der Waals equation with a

its mole fraction. Solution : P=KH⋅X⇒PCO2( g)=KH⋅X(CO2)⇒0.01=35×100..

Filo Student Questions For CBSE , Grade 9 , Chemistry

6.3: Van der Waals and Other Gases - Physics LibreTexts