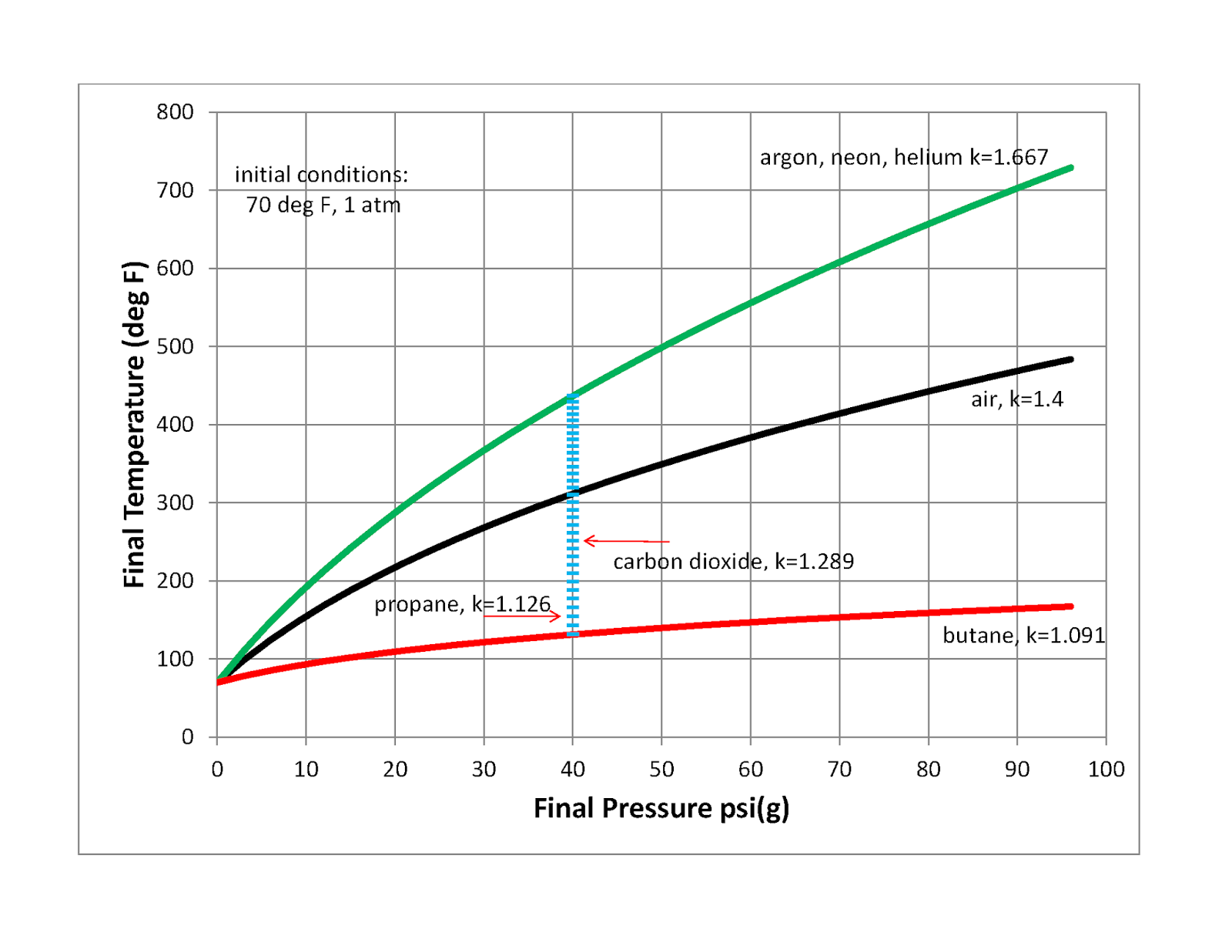
Compression of a gas due to external pressure and the
4.9 (493) · $ 19.50 · In stock

/wp-content/uploads/2021/05/en

Cristian MERINO RUBILAR, Professor (Assistant)

SOLVED: A gas is compressed from an initial volume of 5.55 L to a final volume of 1.24 L by an external pressure of 1.00 atm . During the compression the gas

Waldo QUIROZ, Professor (Full), PhD Chemistry

Q. 2 mole of an ideal gas undergoes isothermal compression along three different paths (i) reversible compression from P; = 2 bar and V; = 4 L to P, = 20 bar (

initialy compressed at same state P 0, V 0, T 0 to half their volumes by three different processI: by the irreversible adiabatic compression against constant external pressure. II: by the isothermal
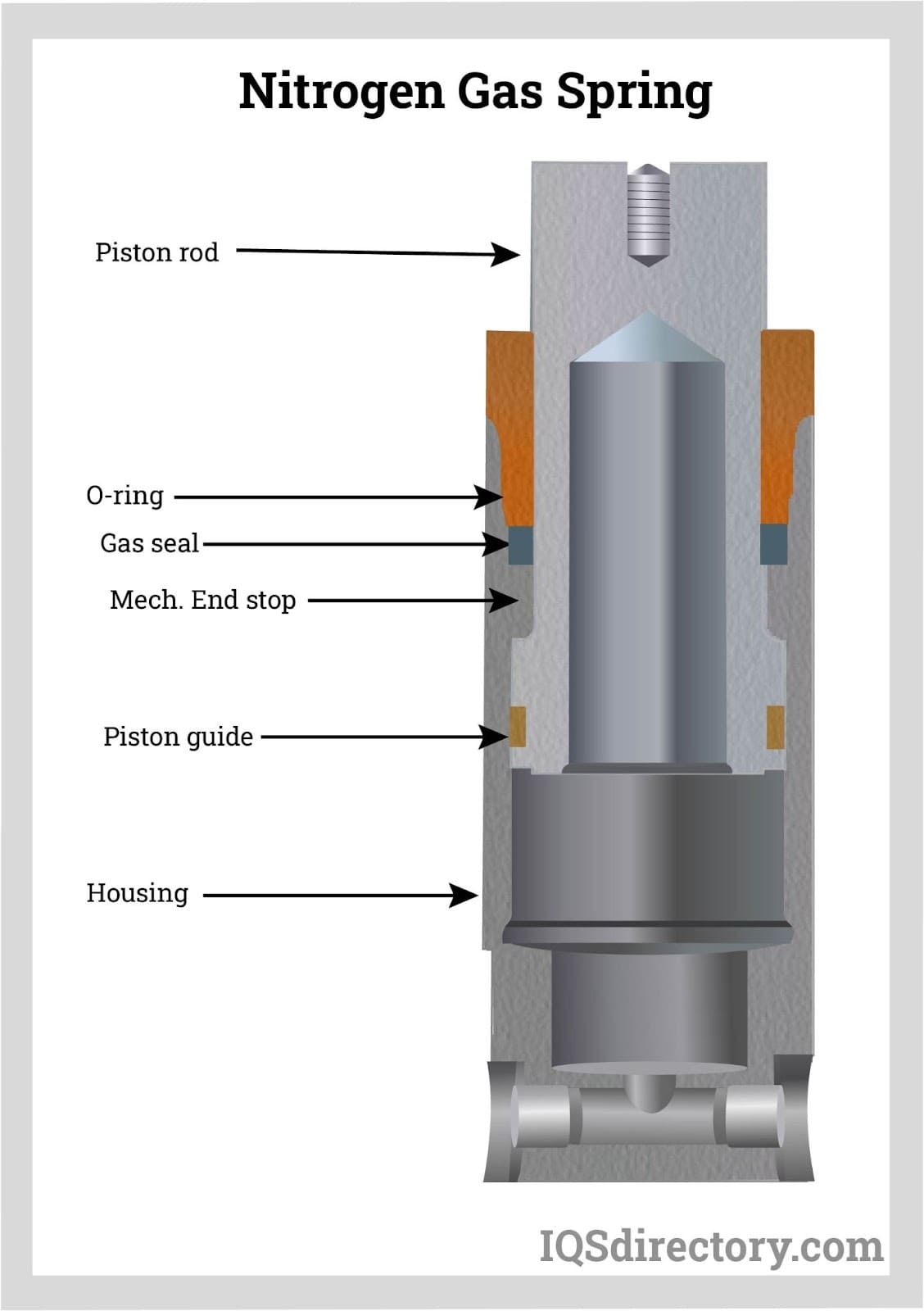
Gas Springs: Types, Design, Benefits, and Applications

What will be the work done on an ideal gas enclosed in a cylinder, when it is compressed by a constant external pressure, pext in a single step as shown in Figure
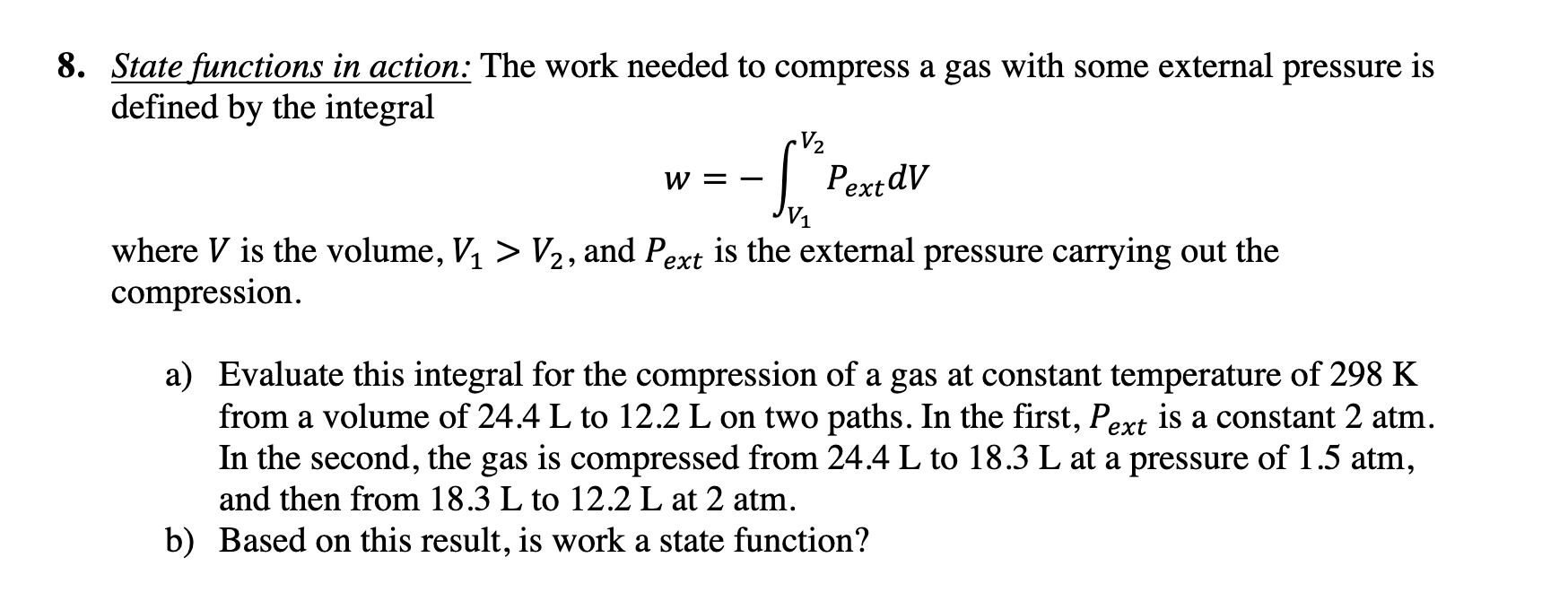
Solved 8. State functions in action: The work needed to
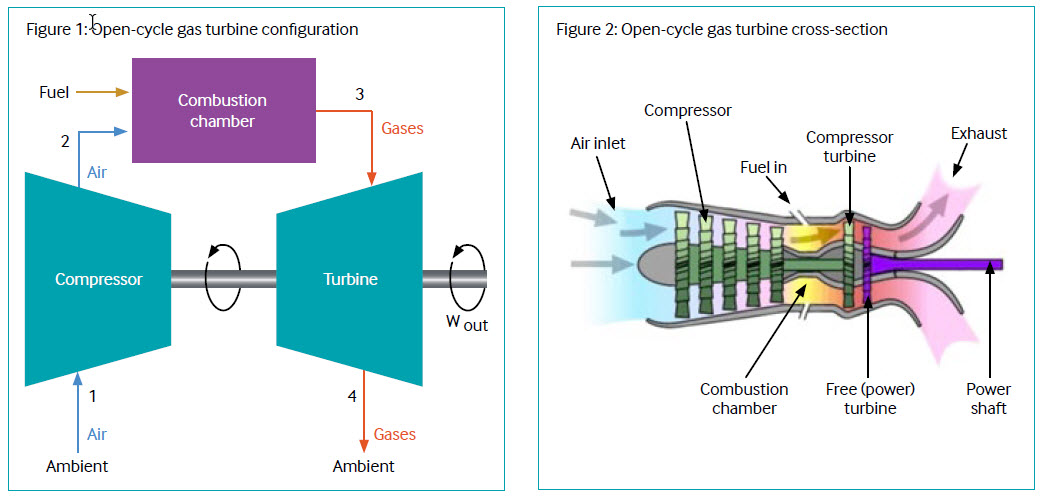
Open-cycle gas turbines (2022)

the constant external pressure required to compress 1 mole of an ideal gas from 23 10^-3m^3to 8 10^-3m^3 when work - Chemistry - Thermodynamics - 16657061

An ideal gas undergoes isothermal compression from 5 m3 to 1 m3 against a..