200 g of a sample of limestone liberates 66 g of CO2 on heating
4.8 (263) · $ 9.00 · In stock
200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating- The percentage purity of CaCO3 in the limestone is Options-a- 95

58. 50 g of a sample of limestone (CaCO3) on complete 58 decomposition gives 20 g of CO2. The percentage purity of CaCO3 in limestone is (Atomic mass of Ca = 40 u) (1) 75% (2) 85% (3) 95.2% (4) 90.9% 0
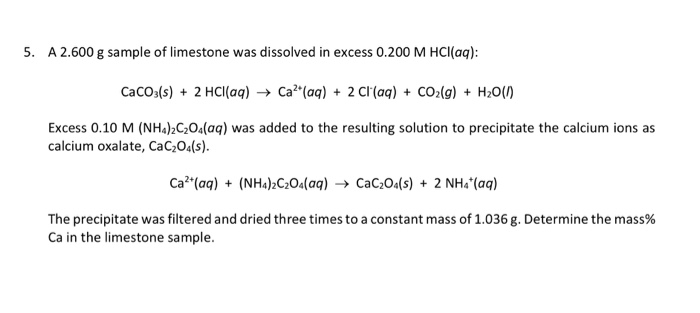
Solved A 2.600 g sample of limestone was dissolved in excess

Modern inorganic chemistry

Carbonate geochemistry and its role in geologic carbon storage - ScienceDirect
Chemistry Class 10 part 1

0958 ch11.pdf - Index of - Free

4) 15 g 8. 50 g of a sample of limestone (CaCO3) on complete decomposition gives 20 g of CO2. The percentage purity of CaCO3 in limestone is (Atomic mass of Ca =

Solved 6 Calcium carbonate (limestone) decomposes when
36. 1.25 g of sample of limestone on heating gives 0.44 g carbon

1996 2009 Kcse Chemistry 1, PDF, Chlorine
PDF) Chemical weathering and atmospheric carbon dioxide (CO2) consumption in Shanmuganadhi, South India: evidences from groundwater geochemistry











