- Home
- pressure compression
- Why do pressure and temperature increase during the compression of a gas? - tec-science
Why do pressure and temperature increase during the compression of a gas? - tec-science
4.5 (471) · $ 19.00 · In stock
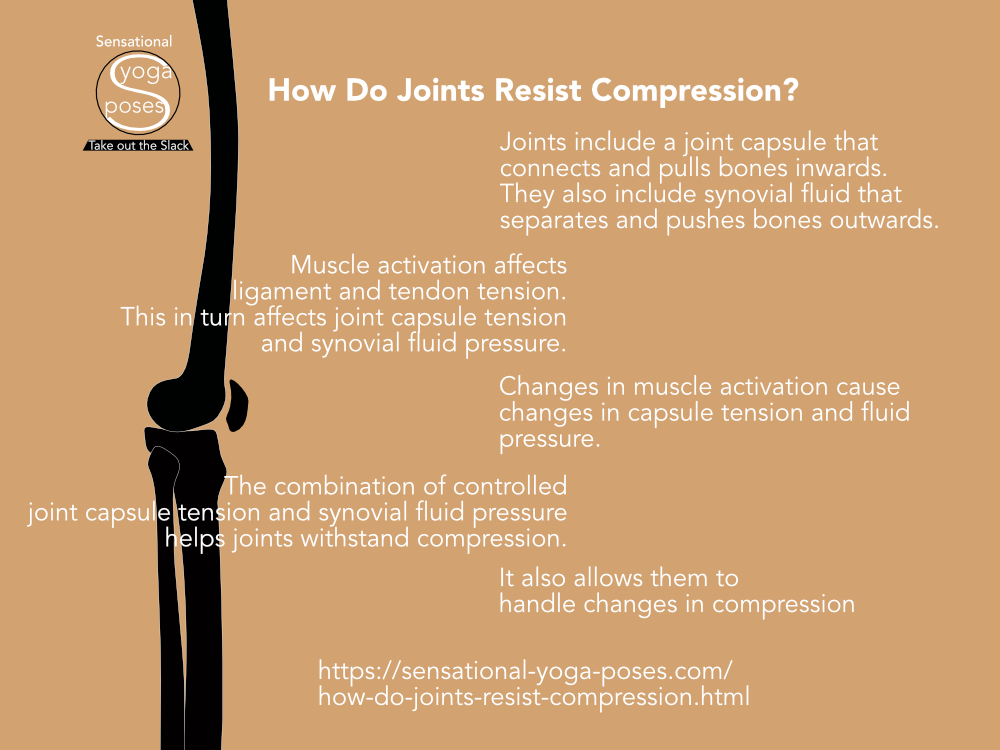
The energy added as work during the compression of a gas leads to an increase in pressure and temperature. Learn more about this in this article.

Why is adiabatic compressed air energy storage yet to become a viable energy storage option? - ScienceDirect

What is phase change? Explained by Thermal Engineers

The Refrigeration Cycle - In easy to understand descriptions & diagrams!

Why do pressure and temperature increase during the compression of a gas? - tec-science

Why do pressure and temperature increase during the compression of a gas? - tec-science

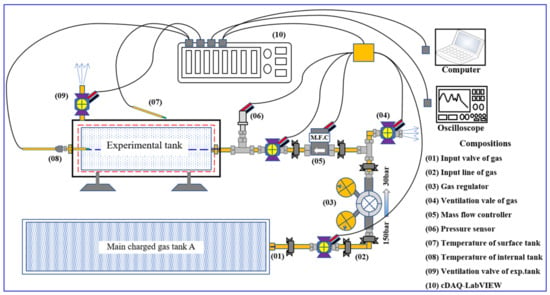
Upgradation of methane in the biogas by hydrogenation of CO2 in a prototype reactor with double pass operation over optimized Ni-Ce/Al-MCM-41 catalyst
Applied Sciences, Free Full-Text

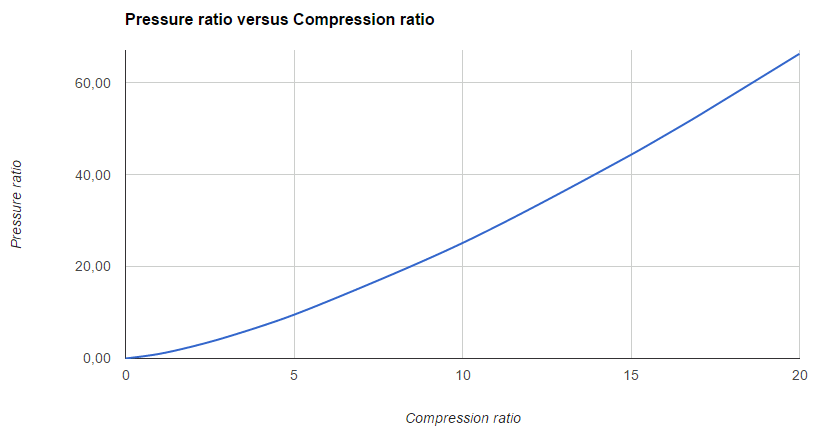
Comparison between air and hydrogen compression gases, showing the
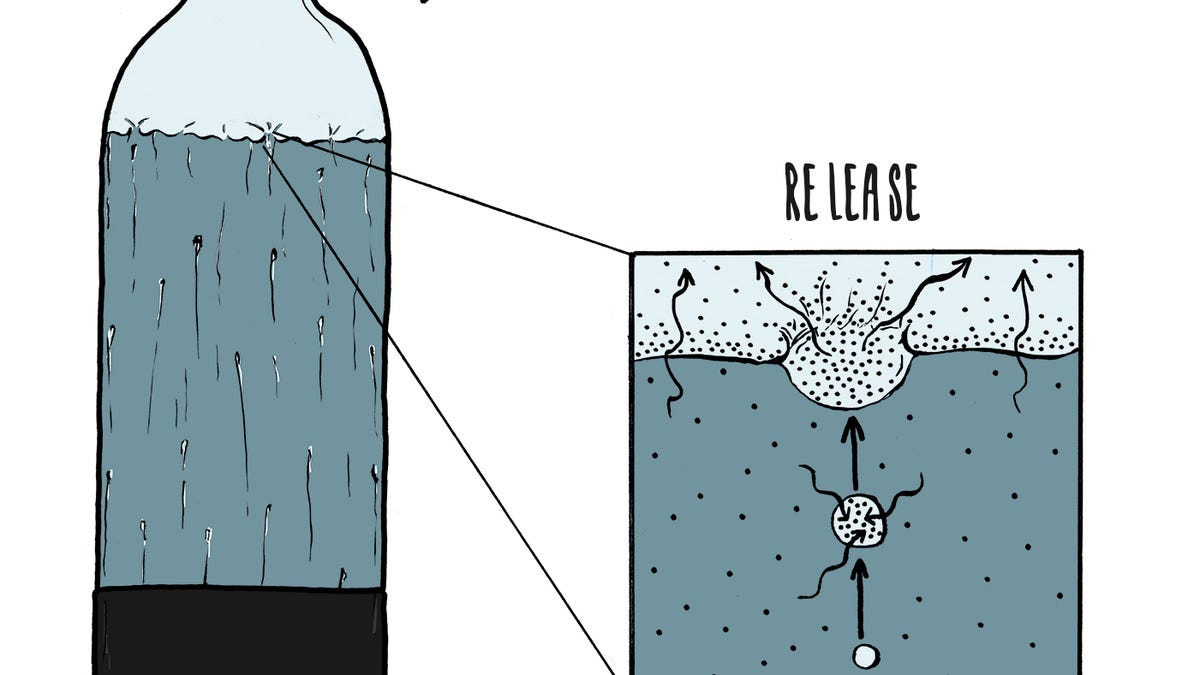
Appliance Science: The compressed chemistry of carbonation - CNET

History and Future of the Compressed Air Economy
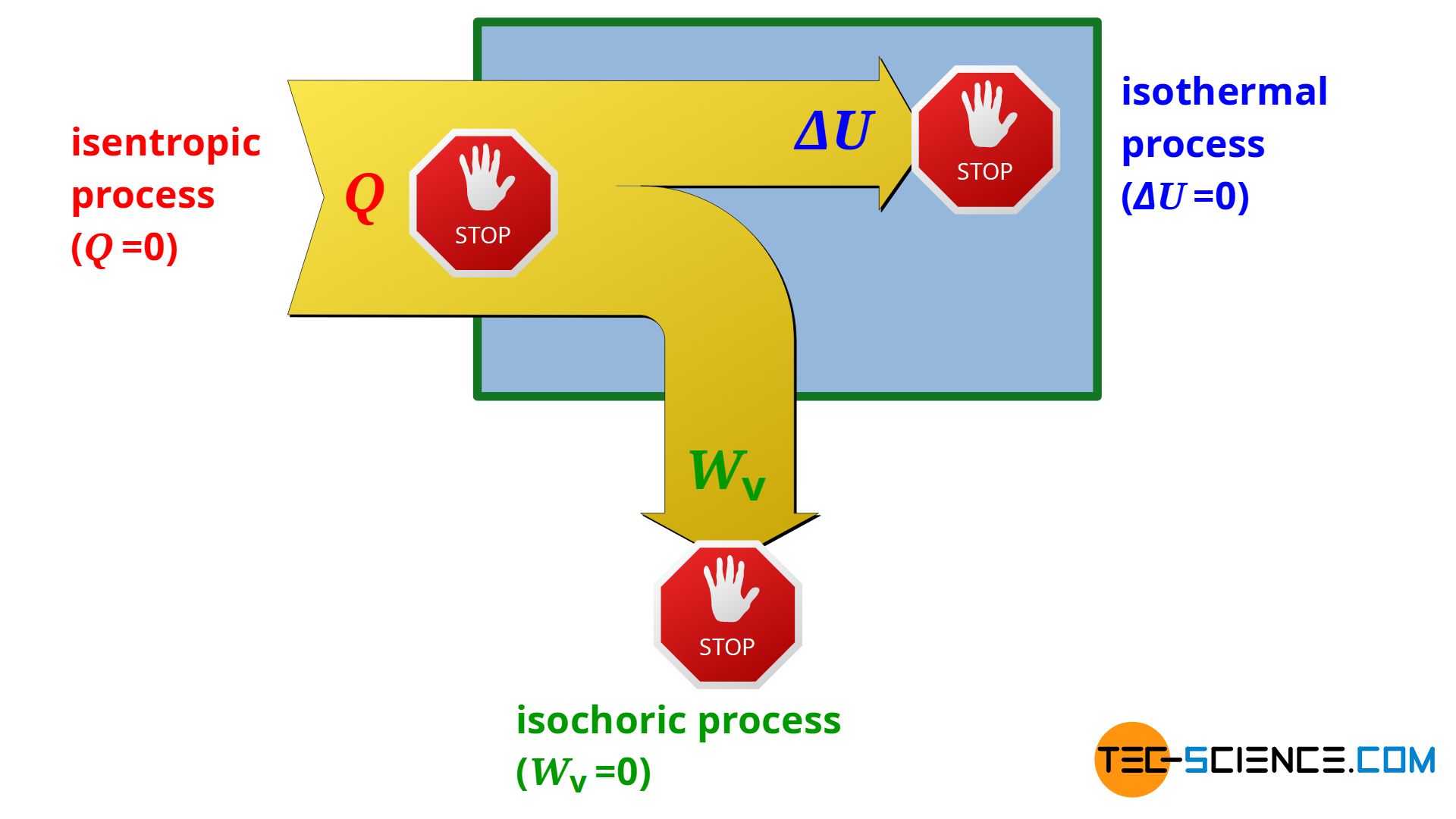
Isentropic (adiabatic) process in a closed system - tec-science
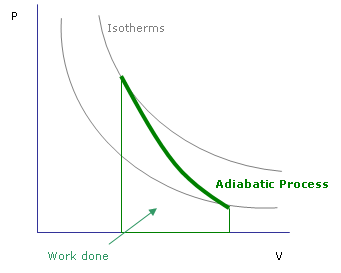
Thermodynamics 101: Compression increases temperature.
High Pressure Processing (HPP) Advantages - Hiperbaric