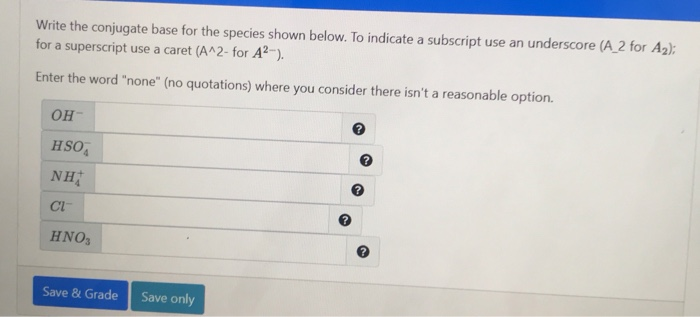
Solved Write the conjugate base for the species shown below
4.9 (235) · $ 30.50 · In stock

Complete a net ionic equation for each proton-transfer reaction

Superacid - an overview

Analysis of Answers to Assignment on Brønsted-Lowry Theory
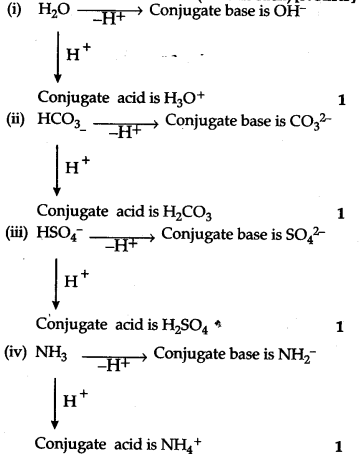
The species${{H}_{2}}$O, H${{CO}_{3}}$, HS${{O}_{4}}$ and N${{H}_{
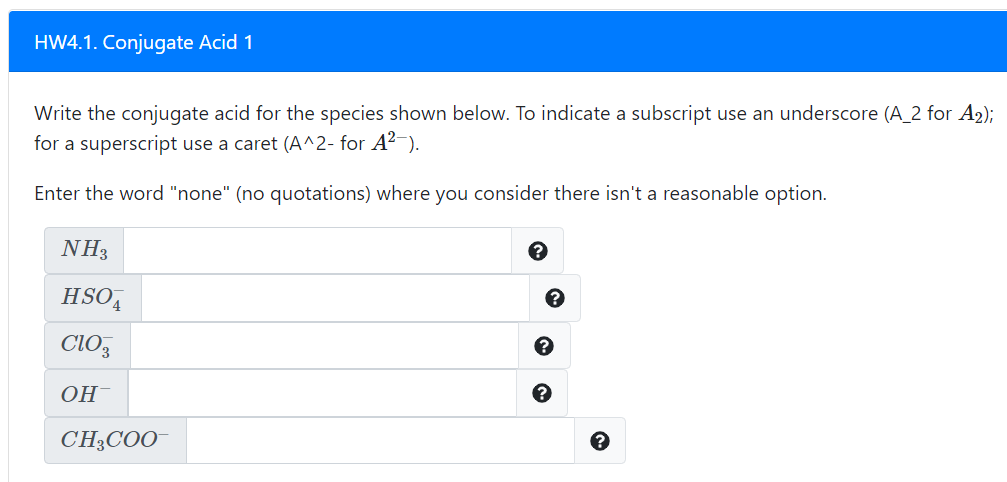
Solved HW4.1. Conjugate Acid 1 Write the conjugate acid for
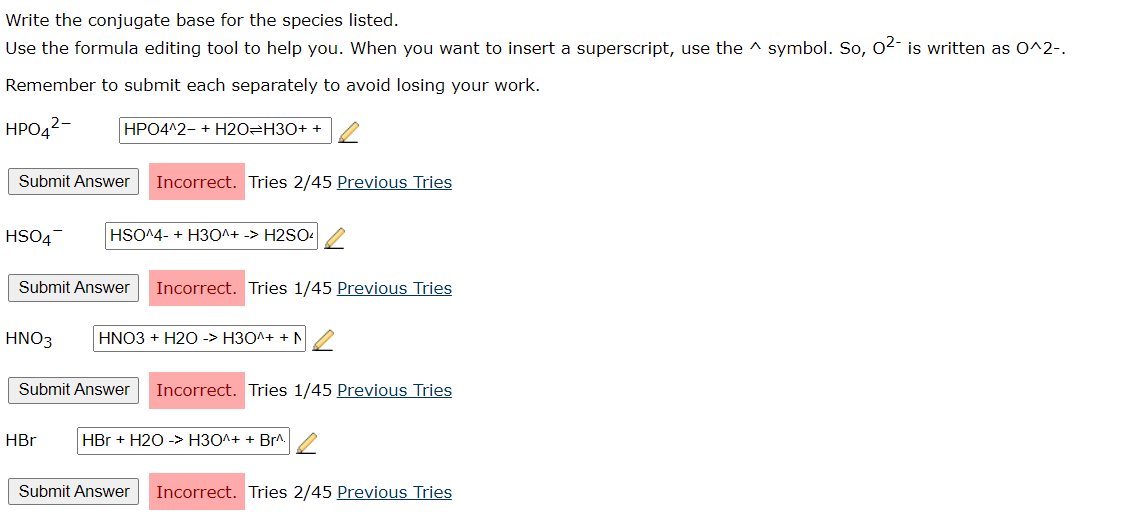
Solved Write the conjugate base for the species listed. Use
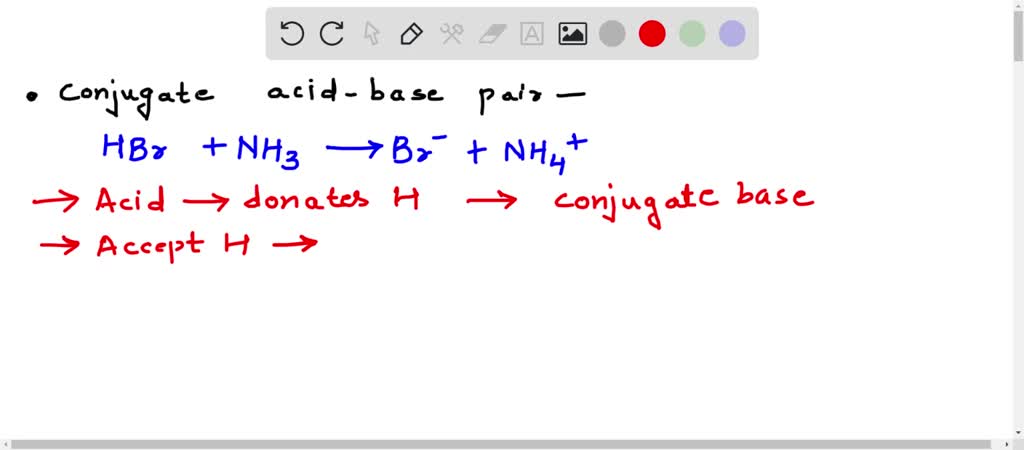
SOLVED: Identify the conjugate acid-base pairs in the reaction
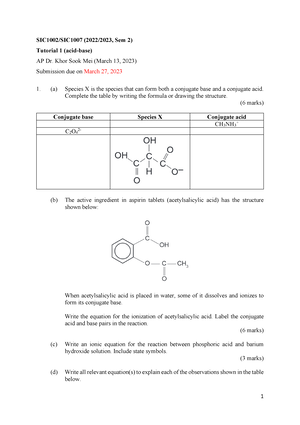
Tutorial 1 (SIC1002 SIC1007) (20222023 Sem 2) - 1 SIC1002/SIC1007
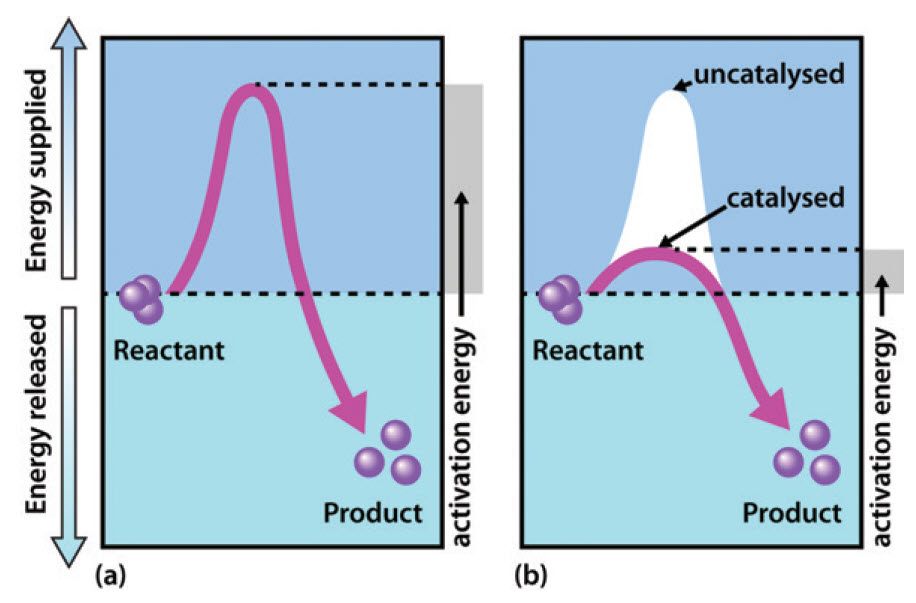
Chapter 7: Catalytic Mechanisms of Enzymes - Chemistry

Brønsted-Lowry acids and bases (article)
You may also like





Related products



© 2018-2024, bellvei.cat, Inc. or its affiliates