physical chemistry - Is the compressibility factor smaller or
4.6 (482) · $ 13.50 · In stock
The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In

Non-Ideal Gas Behavior Chemistry: Atoms First

Physical Chemistry The Compression Factor (Z) [w/1 example

Real gases

Physical Chemistry The Compression Factor (Z) [w/1 example

Compressibility of Liquids - an overview

Compressibility Factor Calculator

Other Causes of Limb Ulcers Causes Physical or chemical injury

Physical Chemistry The Compression Factor (Z) [w/1 example
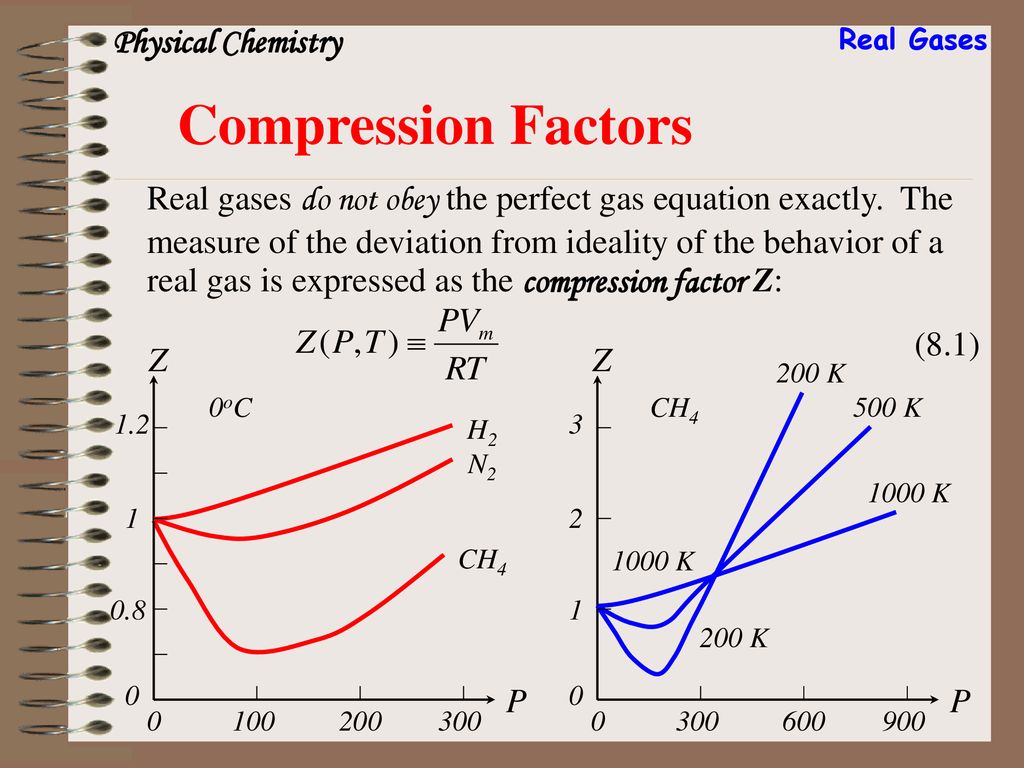
Chapter 8 Real Gases. - ppt download

The compressibility factor `(Z)` of real gas is usually less than

Non-Ideal Gas Behavior Chemistry: Atoms First

Compressibility factor (gases) - Citizendium












