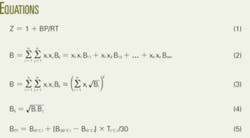- Home
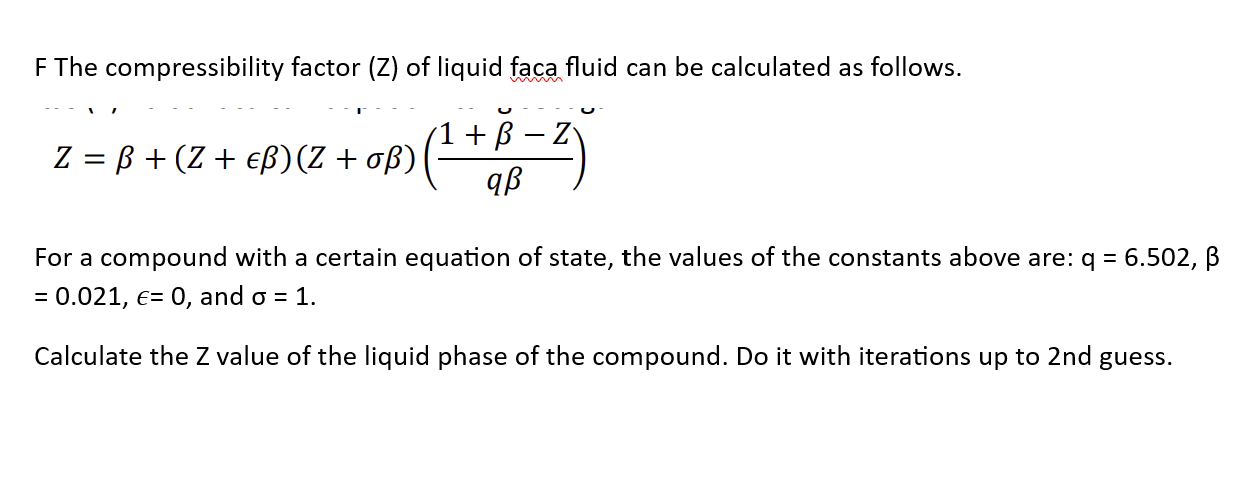
- compressibility factor equation
- What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0
What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0
4.9 (220) · $ 29.99 · In stock

Solved We begin by showing that the compressibility factor

What is the compressibility factor Z for 0.02 mole of a van der waal's gas at pressure of 0.1 atm. Assume the size of gas molecule is negligible. Given: RT =20 L

Compressibility factor - Wikipedia

McMurry and Fay On-Line Chapters

Solved (Triple-Play Bonus) For a certain gas, the

Compressibility factor (gases) - Knowino

Effects of Graphene Oxide Nanosheets and Al2O3 Nanoparticles on CO2 Uptake in Semi‐clathrate Hydrates - Hassan - 2021 - Chemical Engineering & Technology - Wiley Online Library

Real Gases, PDF, Gases

What is the compressibility factor (Z) for 0.02 mole of a van der Waals' gas at pressure of 0.1 a