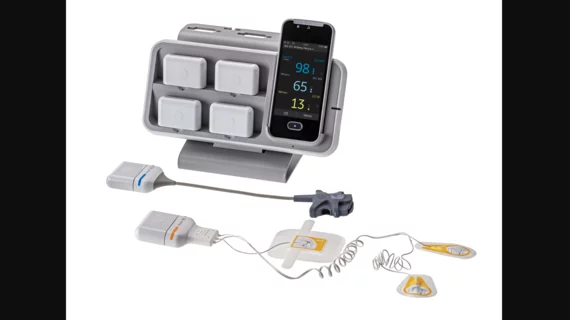
GE HealthCare earns FDA clearance for new remote patient monitoring device
4.7 (225) · $ 7.00 · In stock
The newly cleared device was designed with patient freedom and flexibility in mind.
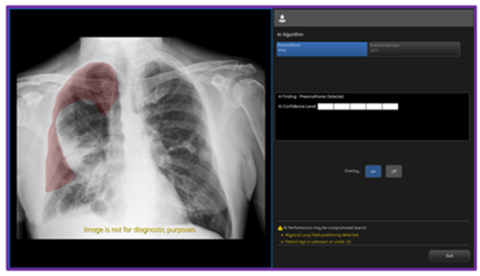
GE HealthCare Expands On-Device Triage Capabilities of Critical Care Suite with FDA Clearance of Algorithm for Pneumothorax Detection, Notification, Triage and Diagnosis -November 28, 2023 at 09:47 am EST

GE Healthcare Expands AI, Digital and Imaging Solutions - ICE

Edison Digital Health Platform, GE Healthcare Launches New Digital Health Platform - Digital Health Times

Device Sectors / Patient Monitoring

Remote Monitoring
Home Health Exec

GE HealthCare wins FDA clearance for new Digital Expert Access - Medical Device Network
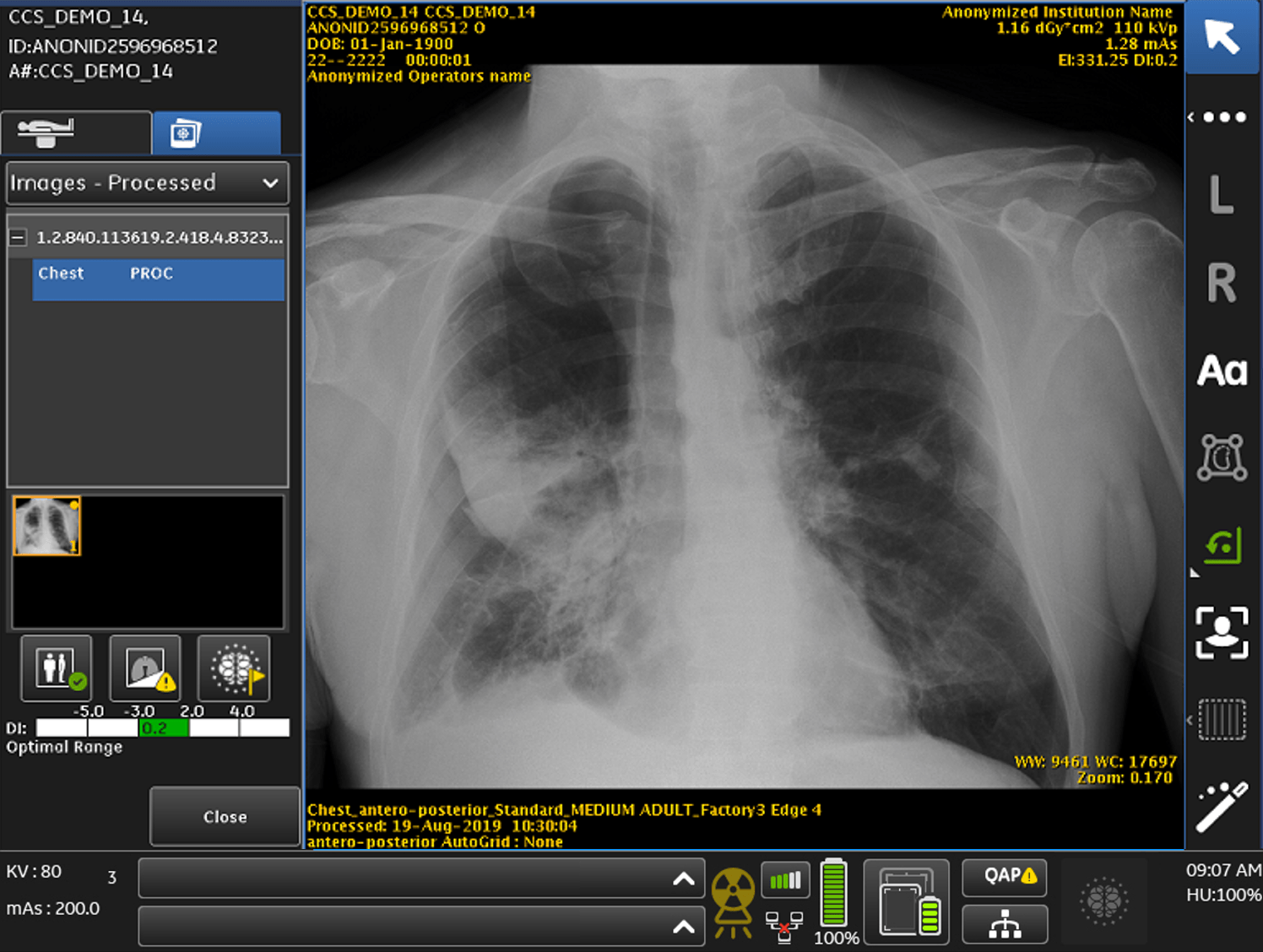
FDA Clears GE Healthcare's AI Algorithms Embedded on Mobile X-Ray Device

Groundbreaking Device Enables 30 Days of Continuous Vital Signs Monitoring
GE HealthCare envisions dynamic post-spinoff future for Milwaukee-area campuses - Milwaukee Business Journal
Butterfly Network Launches Third-Gen Ultrasound with Remote Monitoring Ambitions

Simon Philip Rost on LinkedIn: #gehealthcare #medicalinnovation #aihealthcare #patientcare…
Medical Devices Market Size Estimated to Reach USD 996.93

GE HealthCare to distribute FDA-cleared remote patient support