- Home
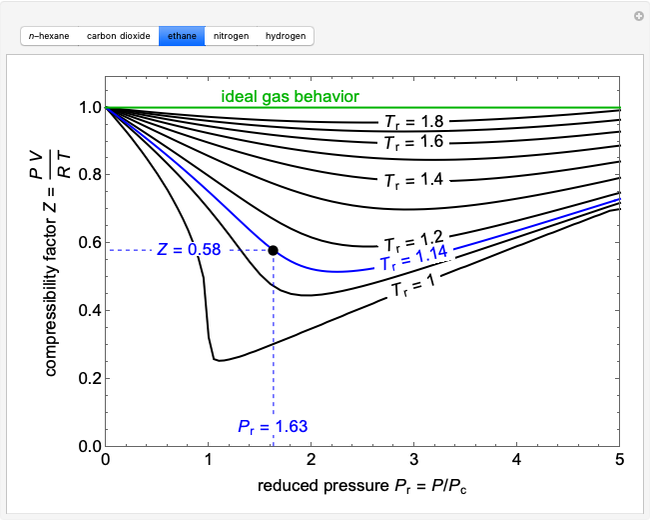
- compressibility factor equation
- The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect
4.7 (135) · $ 13.99 · In stock
The compression factor (compressibility factor) for one mole of a van der Waals

For one mole of a van der Waals gas when b = 0 and T = 300 K , the PV vs 1/V plot is shown below . The value of the

Physical Chemistry The Compression Factor (Z) [w/1 example]

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

States Of Matter Notes: Class 11, JEE, NEET, AIIMS

A gas has a compressibility factor of 0.5 and a molar volume of 0.4 dm3 mol− 1 at temperature of 800K

States Of Matter Notes: Class 11, JEE, NEET, AIIMS

Real gases 1.4 Molecular interactions 1.5 The van de Waals equation 1.6 The principle of corresponding states Real gases do not obey the perfect gas law. - ppt download
The compressibility factor for a real gas at high pressure is (a) 1+RT/pb (b) 1 (c) 1+pb/RT (d) 1-pb/RT - Sarthaks eConnect
A gas described by van der Waals' equation (a) behaves similar to an ideal gas molar volumes - Sarthaks eConnect

States Of Matter Notes: Class 11, JEE, NEET, AIIMS
The density of the vapour of a substance at 1 atm pressure and 500 K is 0.36 kg m^-3. - Sarthaks eConnect


/product/21/3706221/1.jpg?2806)