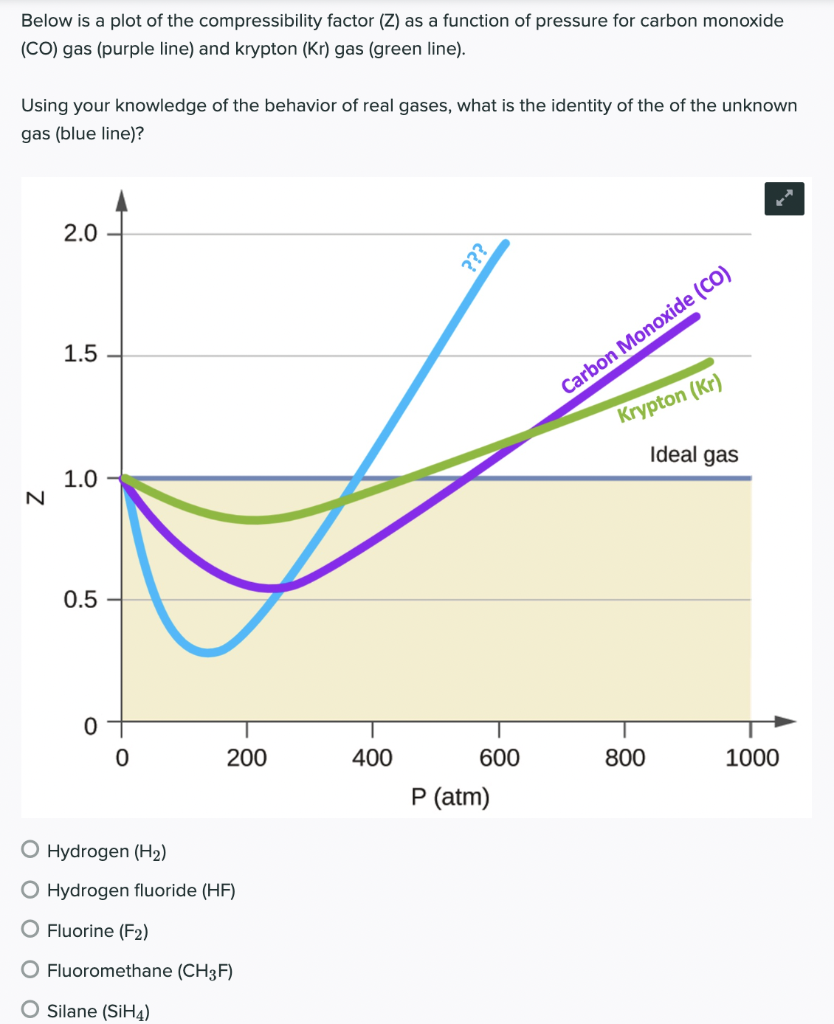
For a given gas, a graph is shown between compressibility factor
5 (547) · $ 21.99 · In stock
By the graph, at T2, the gas does not deviate from ideal gas behaviour at low pressure (Z = 1).Hence, T2 = TBT2 = TB (Boyle's temperature)T1 > TB (Here, Z > 1)This implies that T3 B and TB > TC(Here, Z So, T1 and T2 must be above TC.

The given graph represents the variation of Z (compressibility factor) vs. P three real gases A, B and C. Identify the correct statementFor the gas A, a=0 and its dependence on P

e Compressibility factor (Z) for hydrogen WRT pressure and temperature
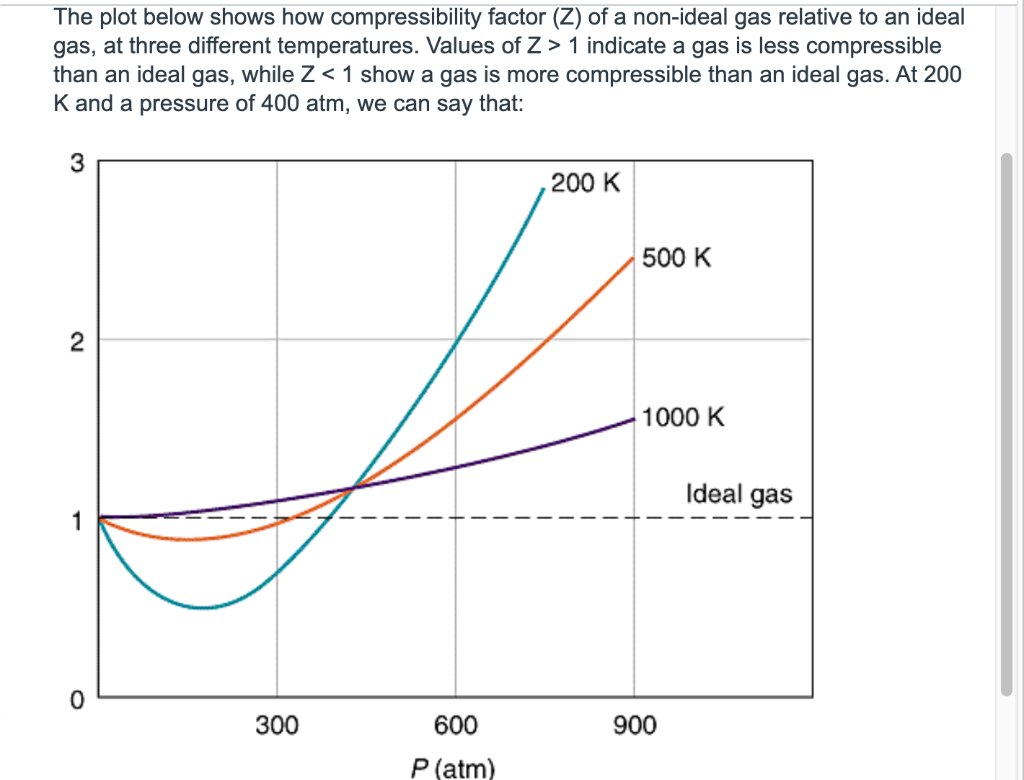
Solved Using the Maxwell-Boltzmann distribution curves

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2

Compressibility factor for methane.

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Standing and Katz's compressibility factor chart
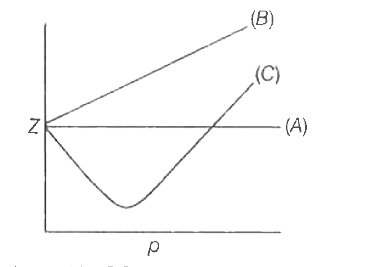
Telugu] The variation of compressibility factor (Z) with pressure (p

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

Real gases

1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts

thermodynamics - Variation of compressiblity factor with temperature - Chemistry Stack Exchange

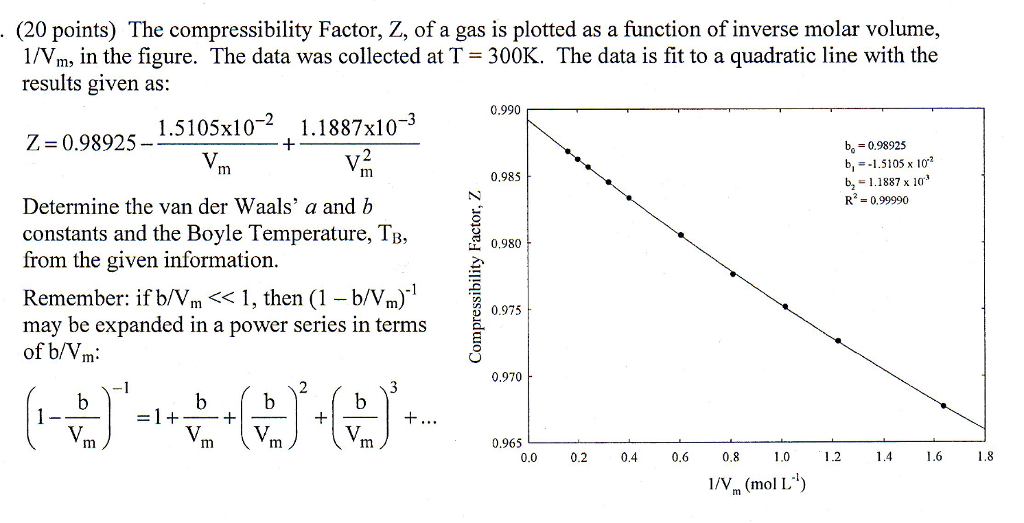
Solved The compressibility Factor, Z, of a gas is plotted as