FDA says Medtronic MiniMed insulin pump recall is serious - MassDevice
4.6 (262) · $ 19.99 · In stock
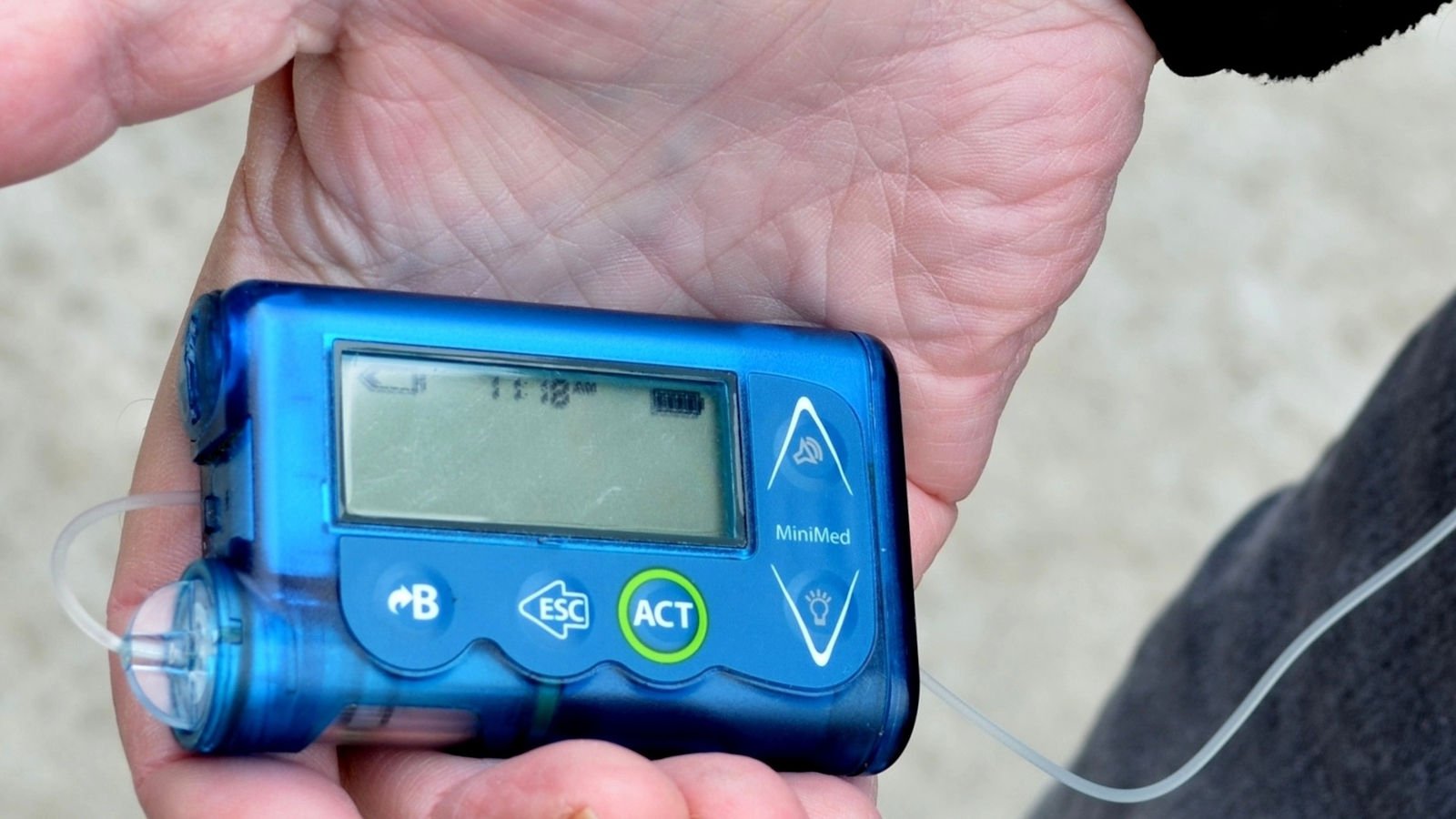
The U.S. FDA has designated a recall of hundreds of thousands of Medtronic Minimed insulin pumps as Class I — the most serious type of recall. Medtronic (NYSE:MDT) first warned of safety problems with the pumps in November. The recall involves 322,005 pumps — MiniMed 630G (model MMT-1715) and MiniMed 670G (model MMT-1780) — in the […]

Medtronic MiniMed Insulin Pump Lawsuit March 2024 - Select Justice

Medtronic expands 2 Class I recalls of MiniMed insulin pumps

Medtronic recalls certain MiniMed insulin pumps tied to 1 death

ICU Medical's Smiths Medical has a Class I infusion pump recall

Medtronic Diabetes Pump Lawsuit - MiniMed Insulin Pump

Diabetes: Medtronic warns on insulin pump overdose
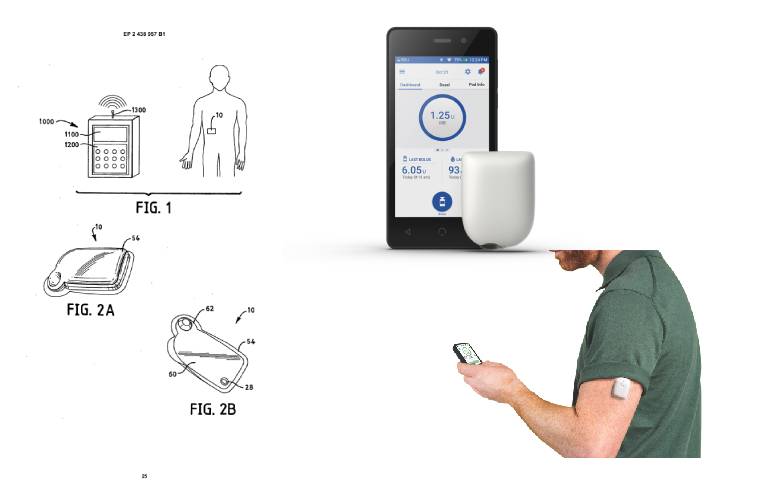
Report: Insulet prevails over Medtrum in France patent spat

Medtronic recalls some insulin pumps as FDA warns they can be hacked

Drug Delivery Business News on LinkedIn: Baxter recalls some syringe pumps due to underdosing risk
Medtronic - Wikipedia
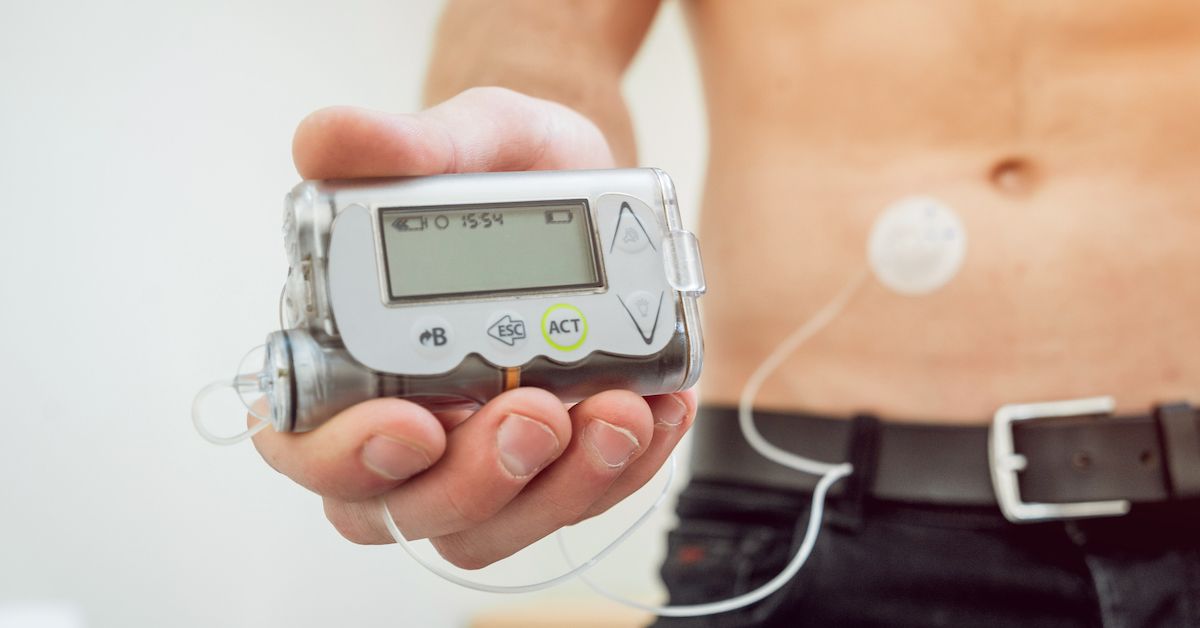
Understanding the Insulin Pump Recall

Insulin pump hacker outs Medtronic - MassDevice
Medtronic Pain Pump SynchroMed II Blamed for Deaths

UPDATE: Medtronic agrees to stop making SynchroMed drug pump