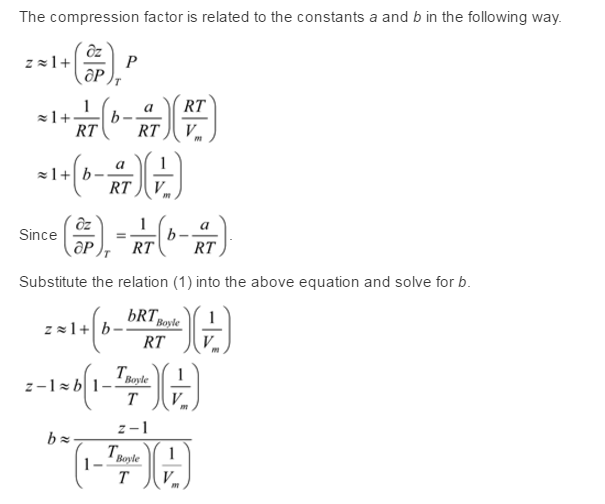
At 273 K measurements on argon gave B = -21.7 cm$^3$ mol$^{
5 (475) · $ 21.00 · In stock

At 273 K and 1 atm pressure, 1 mol of an ideal gas occupies 22.4

Raw images observed at mass 56, with a discharge in argon ͑ a ͒ or

What volume is occupied by 12.5 g of argon gas at a pressure of 1

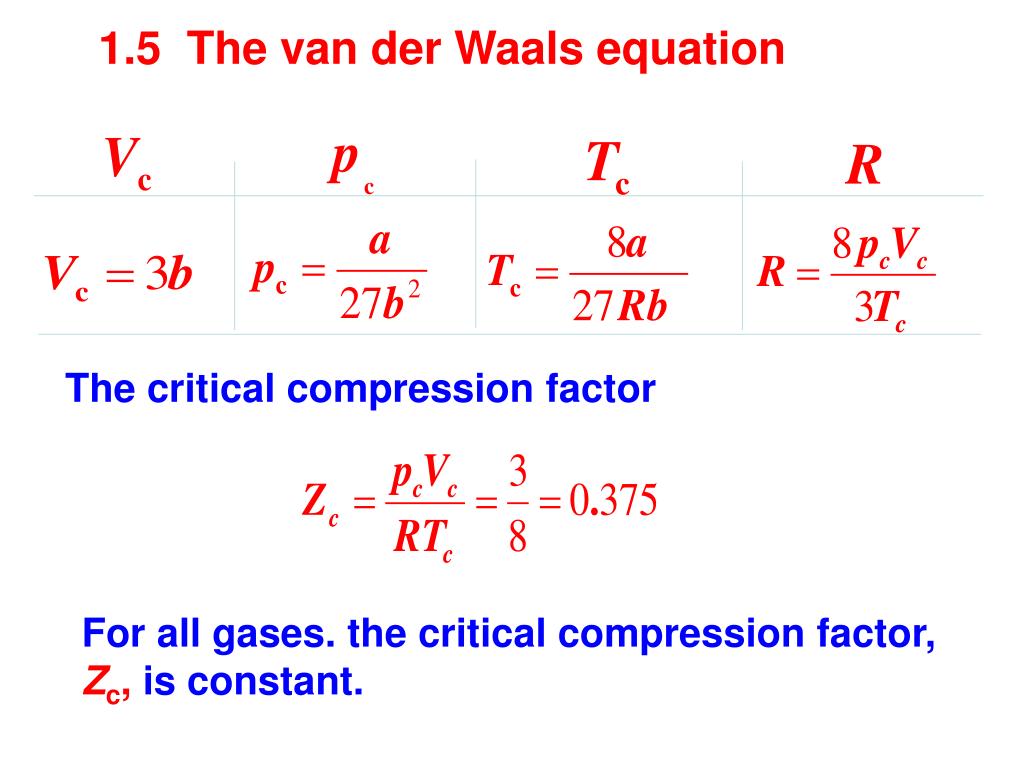
1.2 The kinetic model of gases
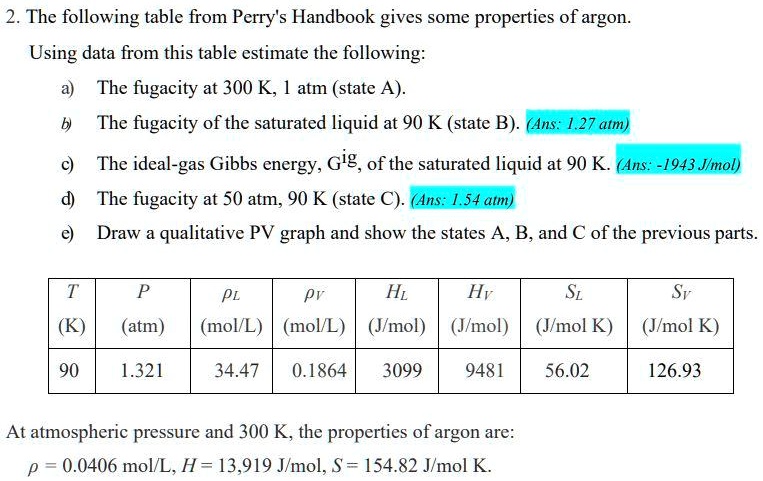
SOLVED: The following table from Perry's Handbook gives some properties of argon. Using data from this table, estimate the following: a) The fugacity at 300 K and 1 atm (state A). b)
6.3: Combining the Gas Laws: The Ideal Gas Equation and the General Gas Equation - Chemistry LibreTexts
-2.png)
Solved] The following financial statements apply

SOLVED: How many moles of Ar are present in 38.7 L at STP? 0 1.83 mol 1.93 mol 1.73 mol none of the given

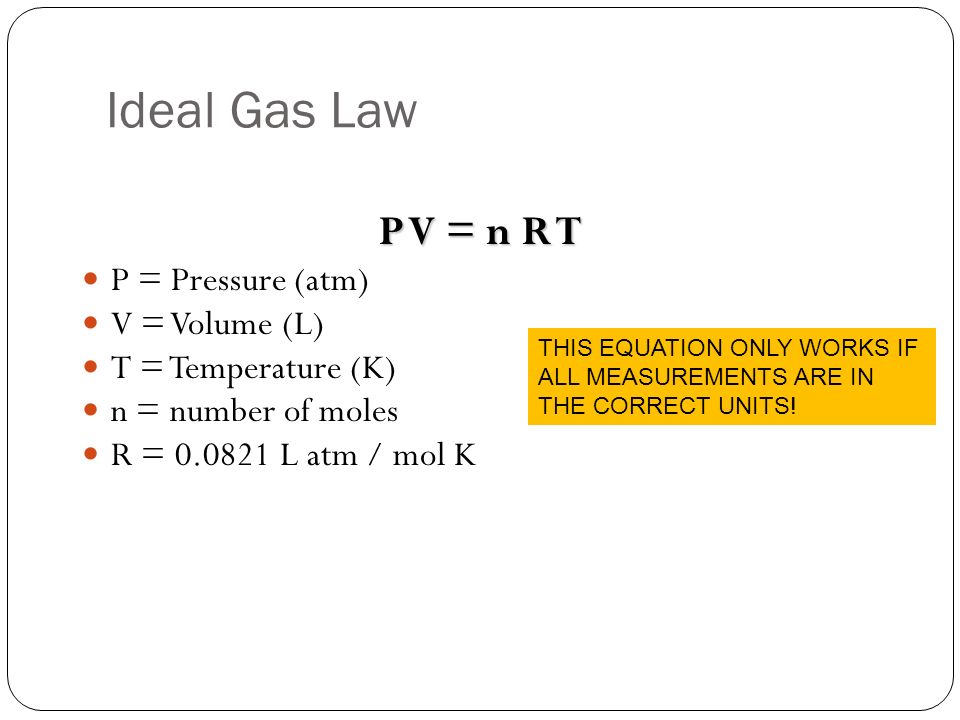
Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law

Dalton's law of partial pressure (article)

McMurry and Fay On-Line Chapters

Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law
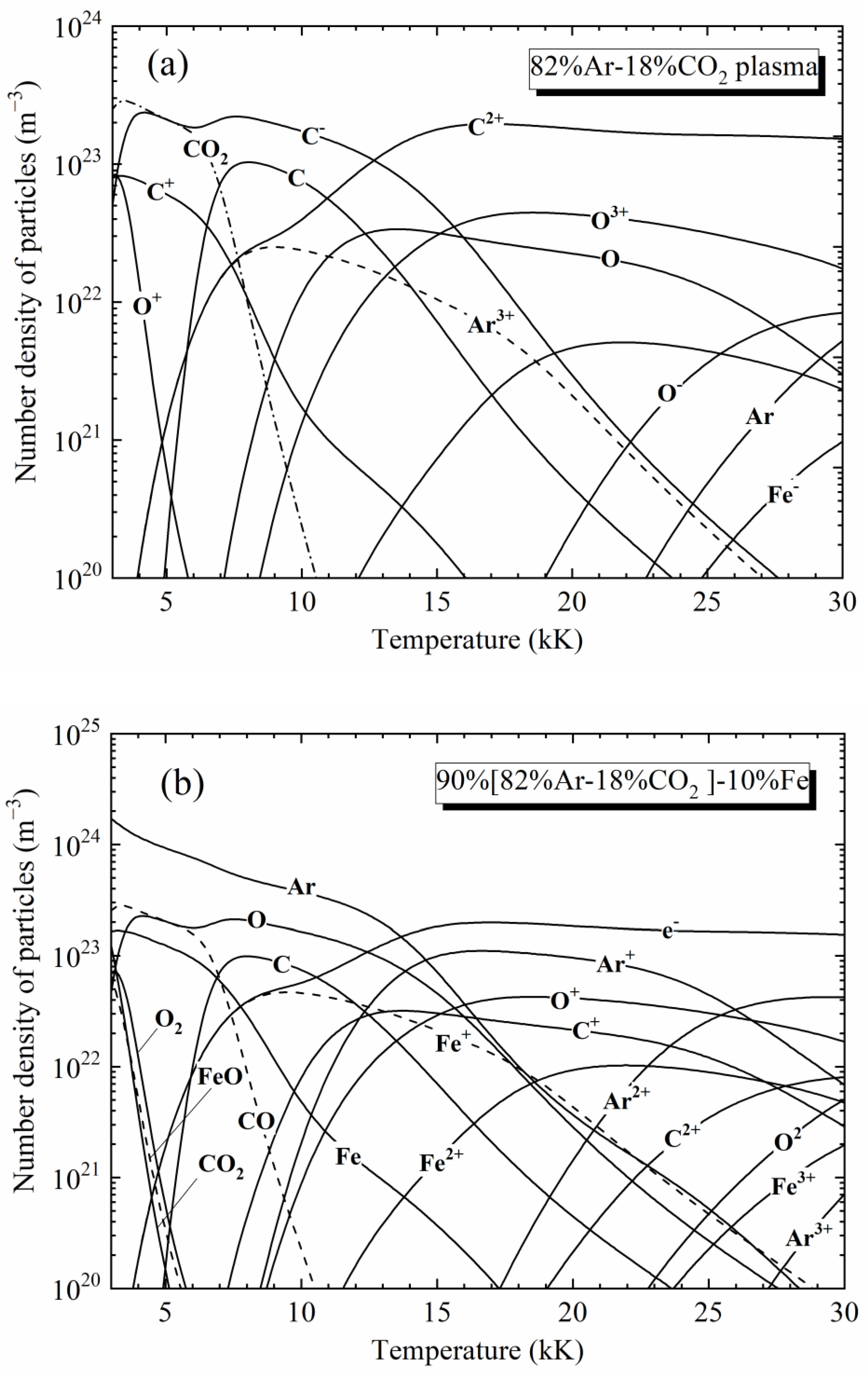
Materials, Free Full-Text
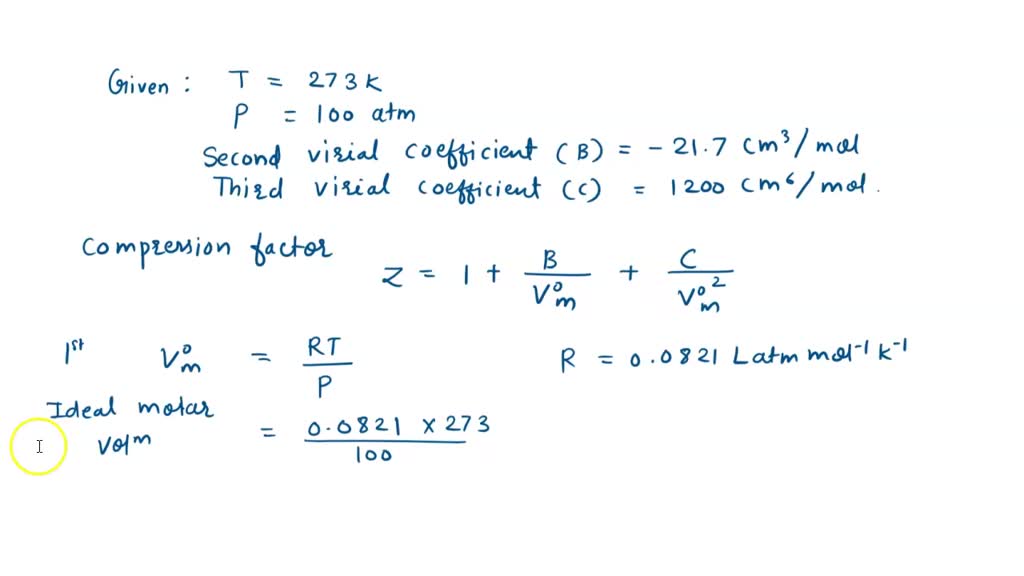
SOLVED: At 273 K, measurements on argon gave B = -21.7 cm^3/mol and C = 1200 cm^6/mol^2, where B and C are the second and third virial coefficients in the expression of
What is the volume occupied by 3.0*10^23 molecules of bromine gas at STP?