- Home
- 42 b
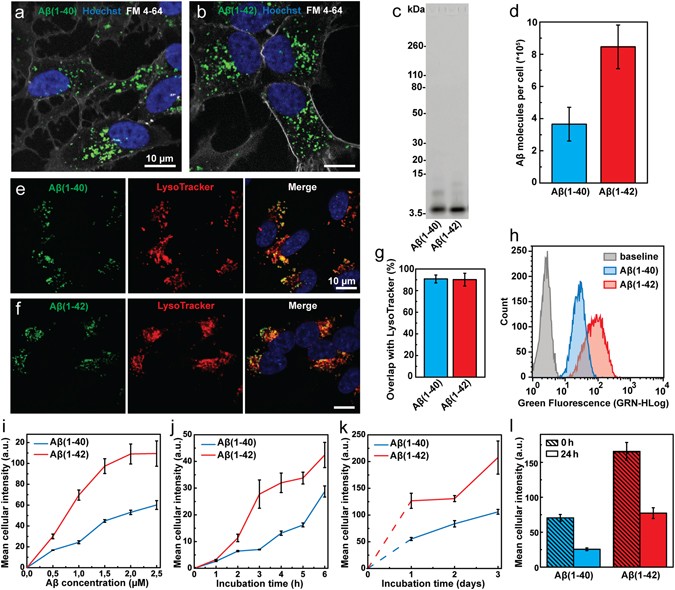
- Endocytic uptake of monomeric amyloid-β peptides is clathrin- and dynamin-independent and results in selective accumulation of Aβ(1–42) compared to Aβ(1–40)
Endocytic uptake of monomeric amyloid-β peptides is clathrin- and dynamin-independent and results in selective accumulation of Aβ(1–42) compared to Aβ(1–40)
4.7 (698) · $ 9.50 · In stock

Cell surface proteoglycan-mediated uptake and accumulation of the Alzheimer's disease peptide Aβ(1–42) - ScienceDirect

The amyloid-β degradation intermediate Aβ34 is pericyte-associated and reduced in brain capillaries of patients with Alzheimer's disease, Acta Neuropathologica Communications

IJMS, Free Full-Text
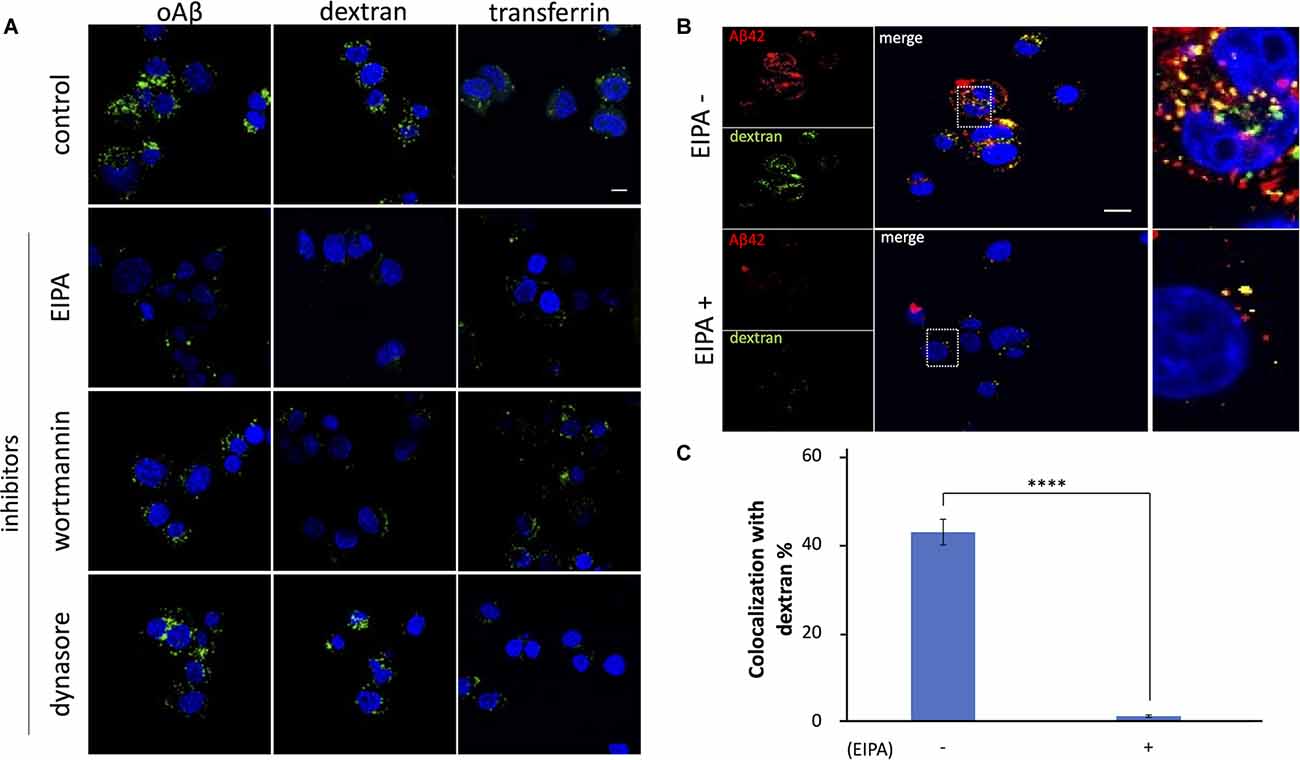
Frontiers Amyloid Beta Is Internalized via Macropinocytosis, an HSPG- and Lipid Raft-Dependent and Rac1-Mediated Process

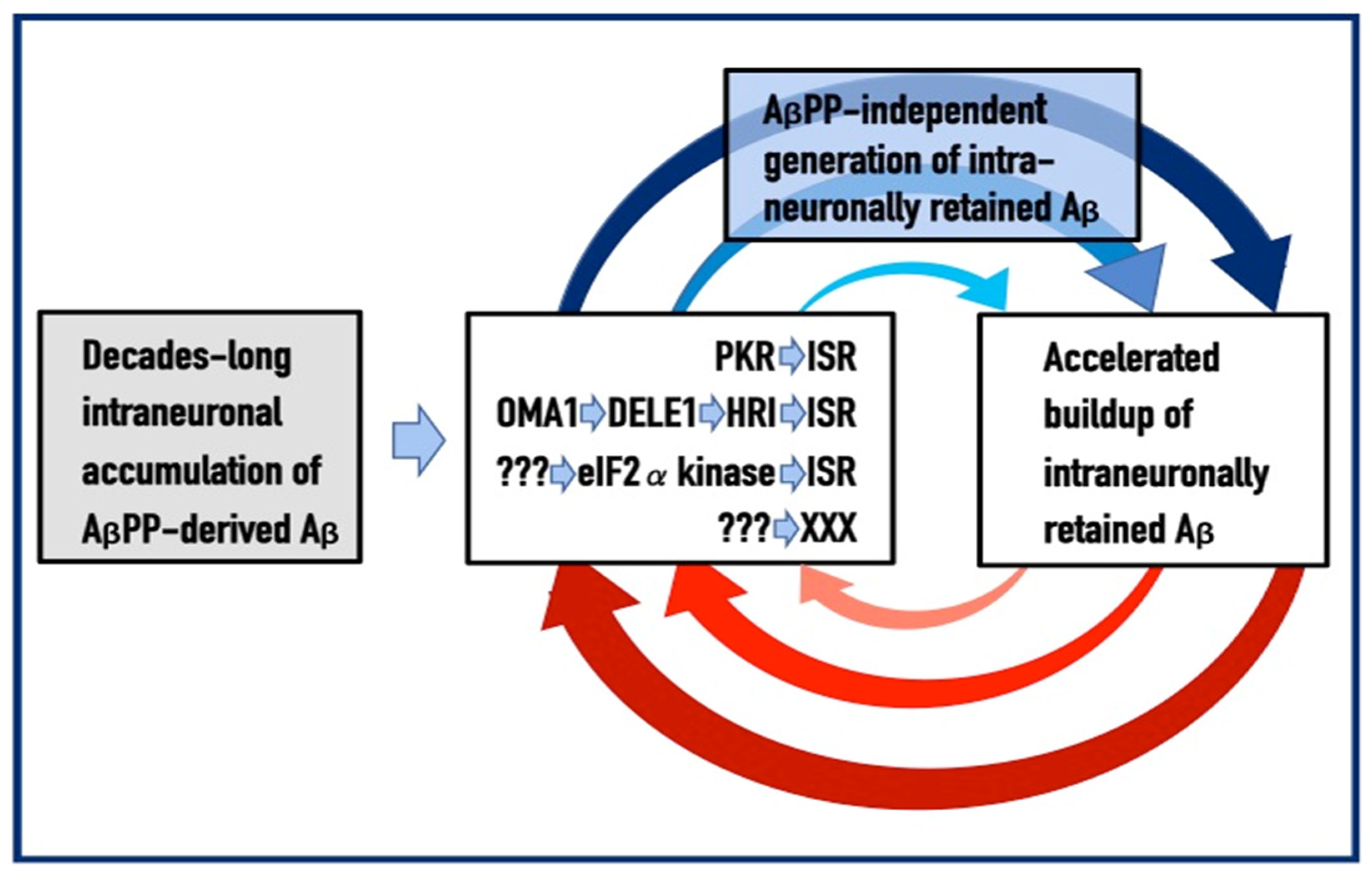
Misfolded amyloid-β-42 impairs the endosomal–lysosomal pathway

The amyloid-β degradation intermediate Aβ34 is pericyte-associated and reduced in brain capillaries of patients with Alzheimer's disease, Acta Neuropathologica Communications
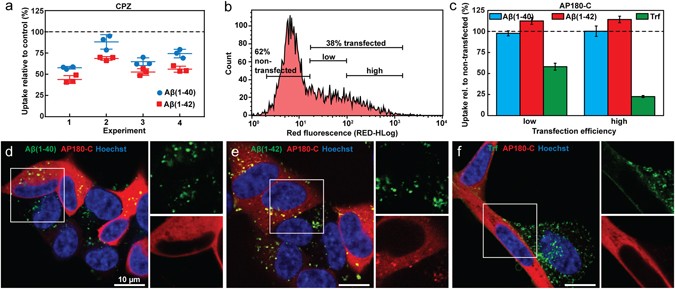
Endocytic uptake of monomeric amyloid-β peptides is clathrin- and dynamin- independent and results in selective accumulation of Aβ(1–42) compared to Aβ (1–40)

In vivo synaptic activity-independent co-uptakes of amyloid β1–42 and Zn2+ into dentate granule cells in the normal brain

Contribution of syndecans to cellular internalization and fibrillation of amyloid-β(1–42)

IJMS, Free Full-Text
IJMS, Free Full-Text
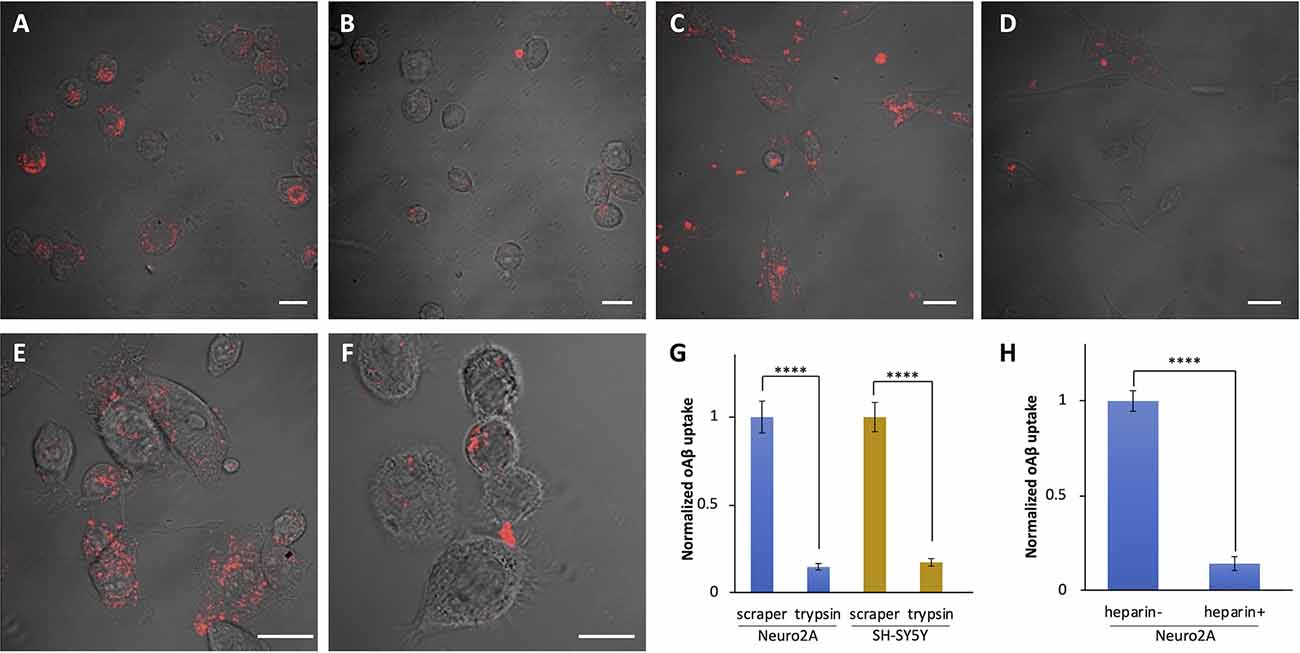
Frontiers Amyloid Beta Is Internalized via Macropinocytosis, an HSPG- and Lipid Raft-Dependent and Rac1-Mediated Process

:max_bytes(150000):strip_icc()/AdobeStock_516075018_Editorial_Use_Only-3140ad22815c49d493cddaef62ff1125.jpeg)