- Home
- compressibility factor equation
- What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
4.8 (137) · $ 18.50 · In stock
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons-an t at different conditions of pressure and volume-Why is Z-1 for H2 and He gas

Compressibility Factor Z Important Concepts and Tips for JEE Main

If Z is a compressibility factor, van der Waals equation at low pressure ..

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

Fluids, Free Full-Text

Solved Problem 2. ( 30 points) The ideal gas law can
How to calculate the characteristic gas constant of a gas (air) - Quora

Solved We begin by showing that the compressibility factor
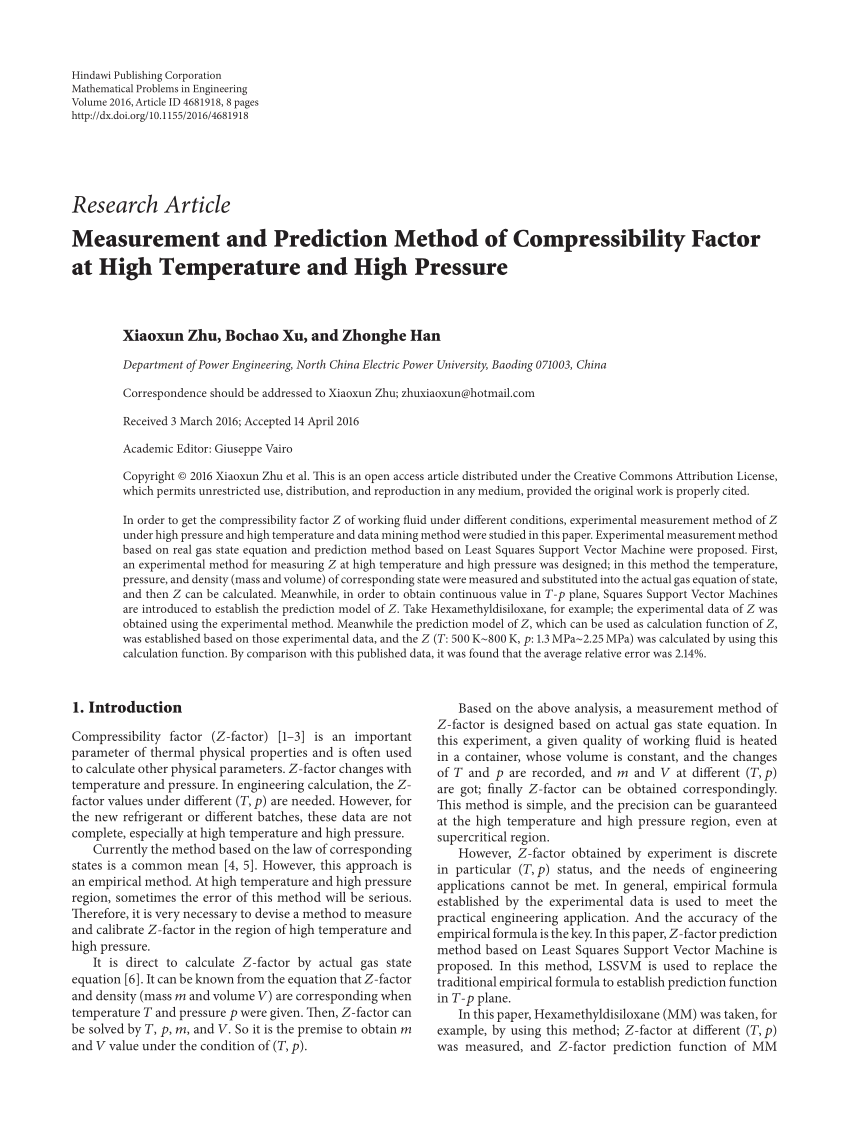
PDF) Measurement and Prediction Method of Compressibility Factor at High Temperature and High Pressure

Real Gases Introductory Chemistry
Real Gases and the Virial Equation

For hydrogen gas, Z > 1 because:value of b is very smallvalue of a is very smallvolume occupied by hydrogen molecules is in comparision with volume occupied by CO_{2}value of b is
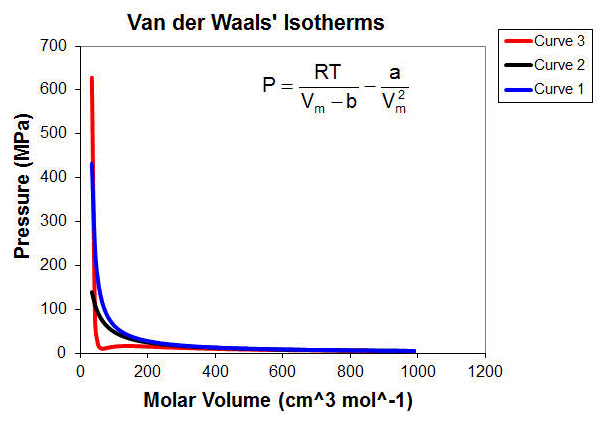
Gas Laws – First Year General Chemistry










