- Home
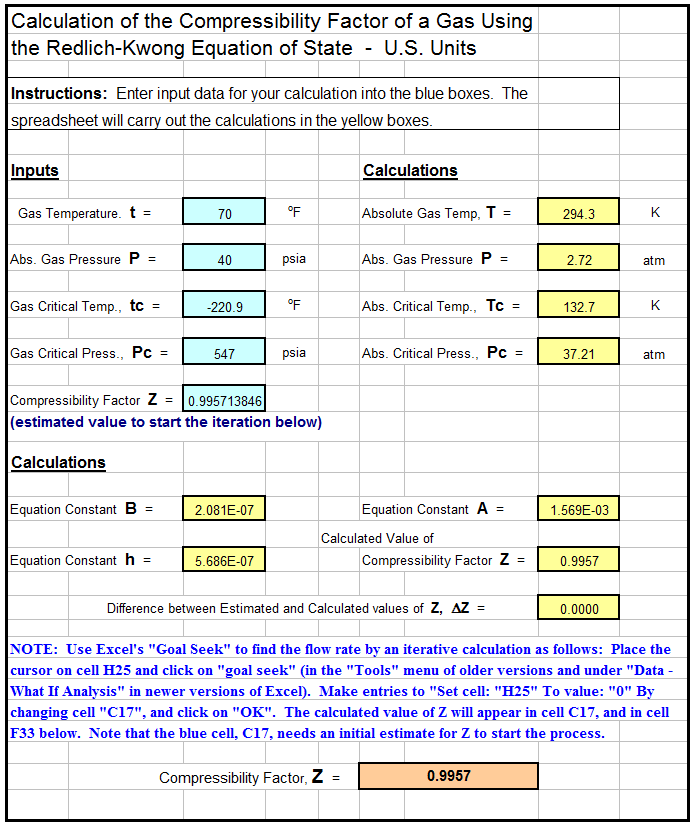
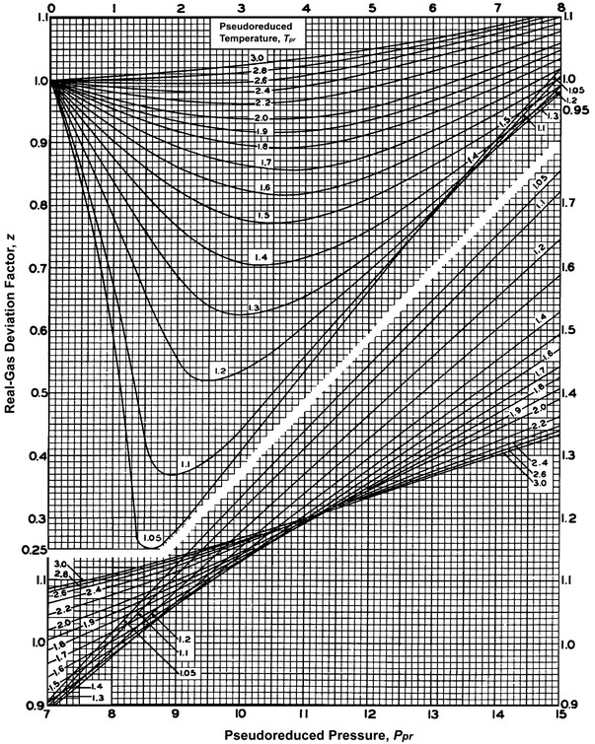
- compressibility factor equation
- the equation of state of a gas is p(v-nb)=rt where b and r are consta - askIITians
the equation of state of a gas is p(v-nb)=rt where b and r are consta - askIITians
5 (790) · $ 6.99 · In stock
the equation of state of a gas is p(v-nb)=rt where b and r are constants. if the pressure and temperature are such that vm=10b what is the value of compressibi
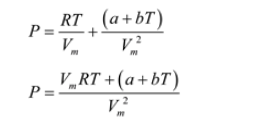
Solved The equation of state of a certain gas is given by p

aieee04
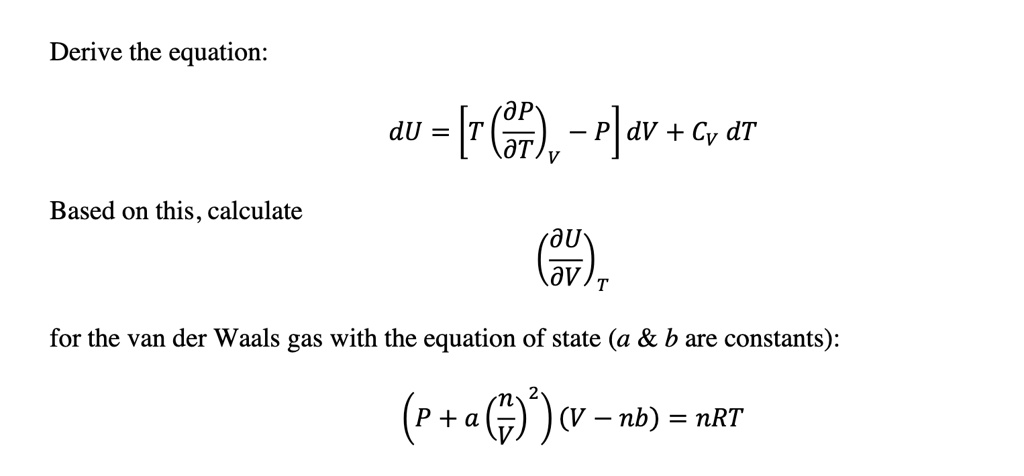
SOLVED: Derive the equation: dU = (6v - P)av + Cv dT Based on this, calculate for the van der Waals gas with the equation of state (a b are constants): (p +

A gas obeys the following equation of state: PV =
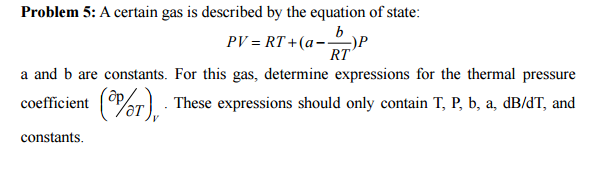
Solved A certain gas is described by the equation of state

SOLVED: The Redlich-Kwong equation of state is given by: RT = a/b * (p + a/( v^2) * (v - b)) where R = the universal gas constant [0.518 kJ/(kg K)], T =
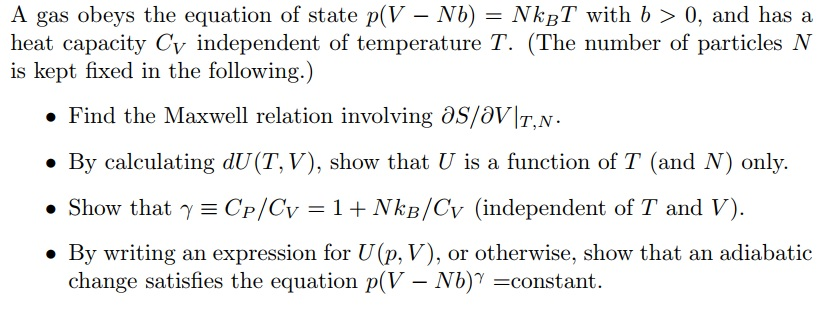
Solved A gas obeys the equation of state p(V-NB) = NkBT with

The equation of state of a gas is given by P + aT 2/ V V c = RT + b , where a , b , c and R are constants.
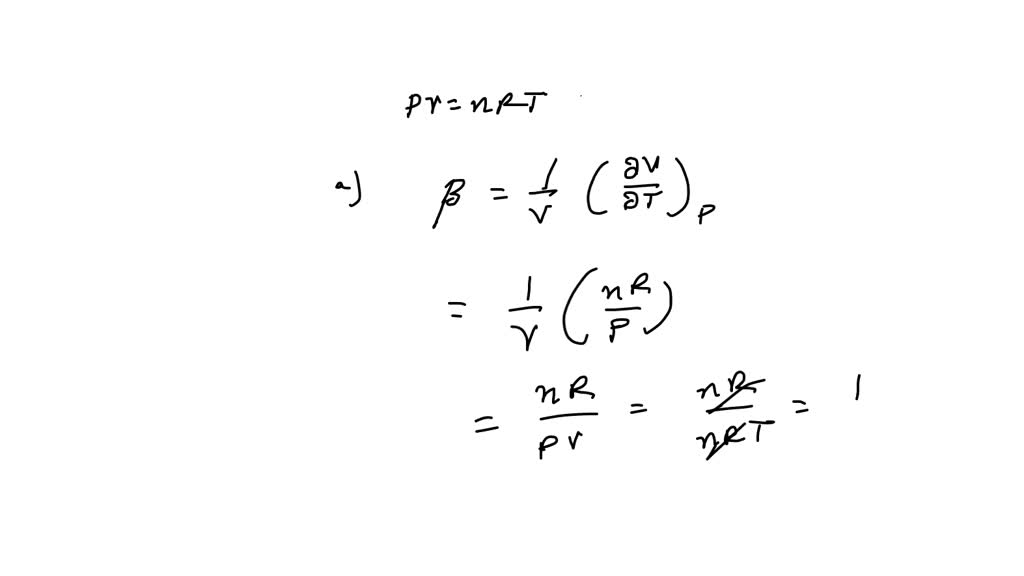
⏩SOLVED:The equation of state of an ideal gas is P V=n R T, where n…

SOLVED: The equation of state of a certain gas is (P + bV) = RT and its specific internal energy is given by U = aT. Find Cv. Show that Cp
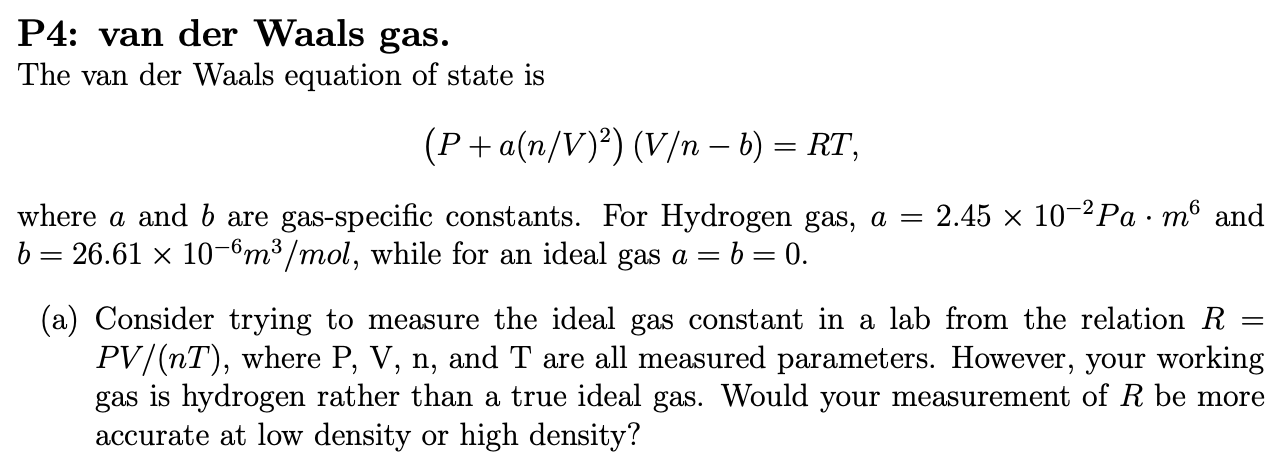
Solved P4: van der Waals gas. The van der Waals equation of

Class – 11. Notes States of matter – ADITYA CAREER INSTITUTE

10 years gate solved papers CHEMISTRY(Upto 2014)

A gas obeys the equation of state `P(V-b) =RT` (The parameter b is a constnat The