- Home
- 80 count
- Calculate the number of protons, electrons, neutrons in Bromine with atomic number 35 and mass number 80.
Calculate the number of protons, electrons, neutrons in Bromine with atomic number 35 and mass number 80.
4.9 (621) · $ 8.50 · In stock
Click here:point_up_2:to get an answer to your question :writing_hand:calculate the number of protons electrons neutrons in bromine with atomic number 35 and mass
Click here👆to get an answer to your question ✍️ Calculate the number of protons- electrons- neutrons in Bromine with atomic number 35 and mass number 80

Composition of an Atom - GeeksforGeeks

Composition of an Atom - GeeksforGeeks

4 Go The atomic masses of two isotopes of O are 15.9936 and 17.0036, calculate in each atom q) number of neutrons, o number of protons, number of electrons, d) mass number.

Calculate the work, in J, if the volume of a system contract

Composition of an Atom - GeeksforGeeks

Calculate the work, in J, if the volume of a system contract

Calculate the number of protons, electrons, neutrons in Brom
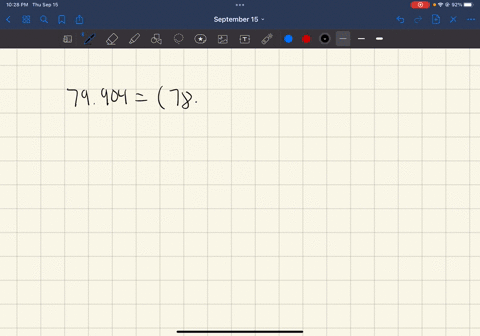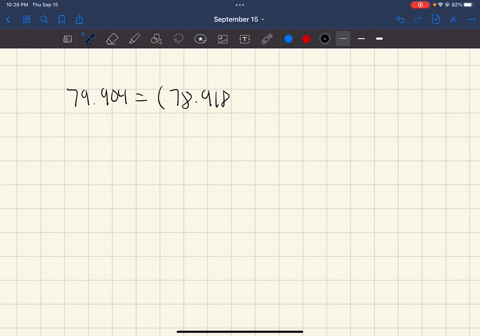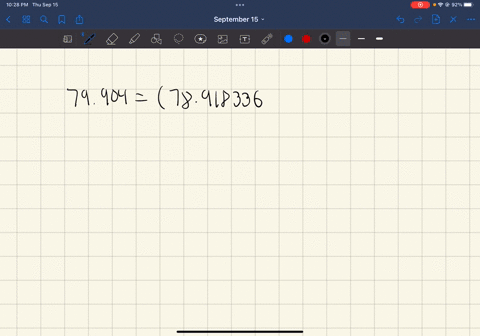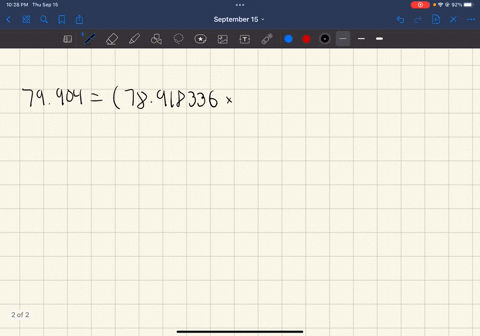
SOLVED: In standard notation, one of the isotopes of bromine is Br-73. The atomic number is 35. How many neutrons does this nucleus contain?
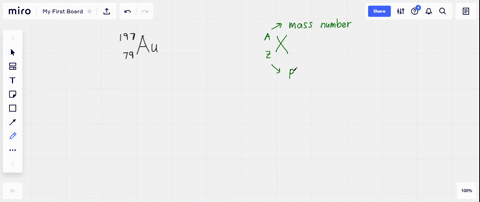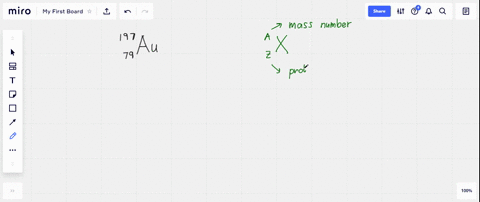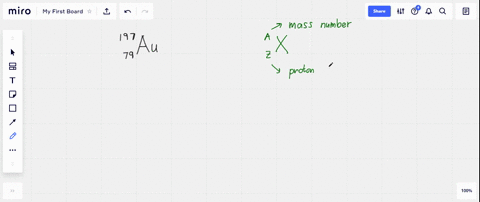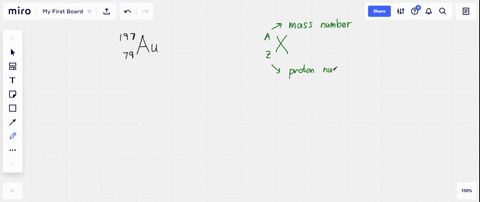
SOLVED: What is the atomic number, mass number, number of electrons, number of protons, and number of neutrons of Bromine-80












