- Home
- 32 g
- 32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
5 (143) · $ 19.50 · In stock
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32- 80 g of h2 is reacted with 80 g of o2 to form water- find out the mass of water obtained-which substance is the limiting reagent

52. 80 g of H, is reacted with 80 g of O, to form water. Find out the mass of water obtained. Which substance is the limiting reagent?
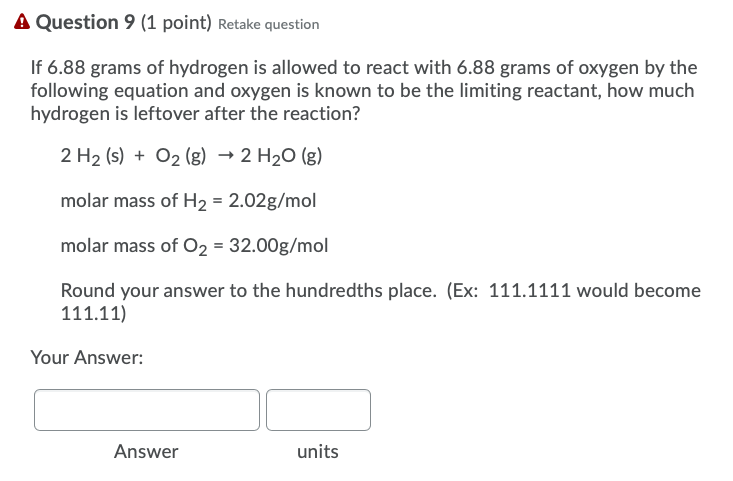
Solved If 6.88 grams of hydrogen is allowed to react with

80 g of `H_(2)` is reacted with 80 g of `O_(2)` to form water. Find out the mass of

Hint: N.(g) + 3H2(9) - > 2NH3(9) 28. 80 g of H, is reacted with 80 g of O, to form water. Find out the mass of water obtained. Whia substance is the limiting reagent?

Solved Hello. Can you please show the step by step solution
How to find Limiting Reagents? - Detailed Explanation with Examples

iii. Mass of mathrm{CO}_{2} remaining =319 mathrm{g} Q.88. 6 mathrm{g} of mathrm{H}_{2} reacts with 32 mathrm{g} of mathrm{O}_{2} to yield water. Which is the limiting reactant? Find the mass of water produced
If 20220 g of H2 and 32 g of O2 reacted to form water, what amount of hydrogen is left behind? - Quora

Aqueous Transformation of a Metal Diformate to a Metal Dihydride Carbonyl Complex Accompanied by H2 Evolution from the Formato Ligands

SOLVED: Question 1: CH4 + 2 H2O → 4 H2 + CO2 Given 80 g of CH4 and 16.3 g of water, what is the limiting reactant?
In the combustion of butane, how many grams of excess water will you have with a reaction between 20.0g of butane and 20.0g of oxygen? - Quora







)


