Solved What is the equilibrium constant (Kp) at 45 °C for
4.7 (102) · $ 9.50 · In stock
Answer to Solved What is the equilibrium constant (Kp) at 45 °C for

chem 112 exam 2 Flashcards
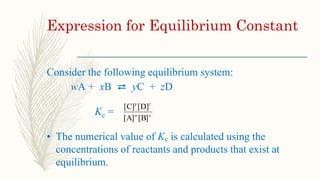
image.slidesharecdn.com/equilibrium-constant-prese

16.41a Calculate the equilibrium constant at 25 °C for O2(g) + 2F2(g) → 2OF2(g) ΔG° = −9.2 kJ

i.ytimg.com/vi/4dKCx2crbG8/maxresdefault.jpg
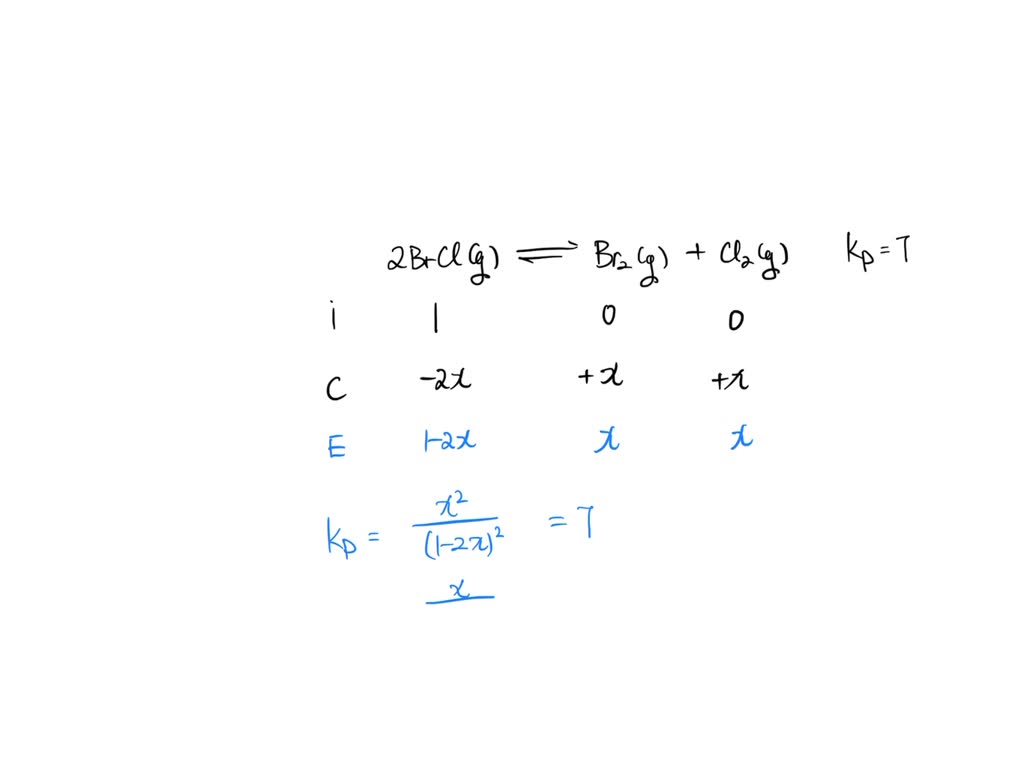
SOLVED: For the equilibrium 2 BrCl (g) Br2 (g) + Cl2 (g), the equilibrium constant Kp is 7.0 at 400 K. If a cylinder is charged with BrCl (g) at an initial
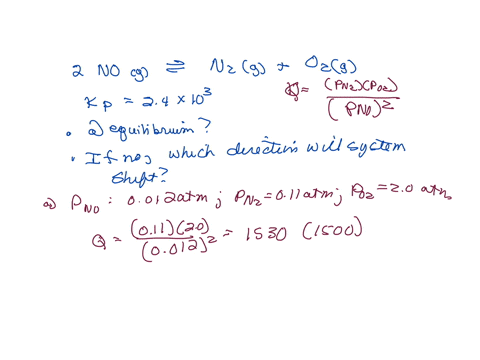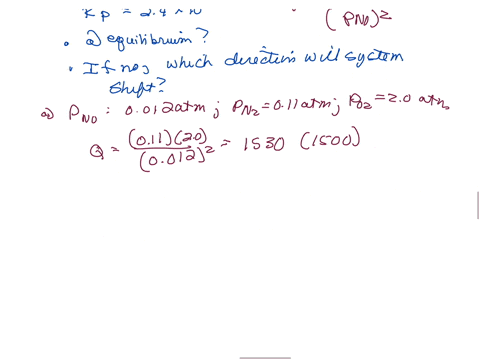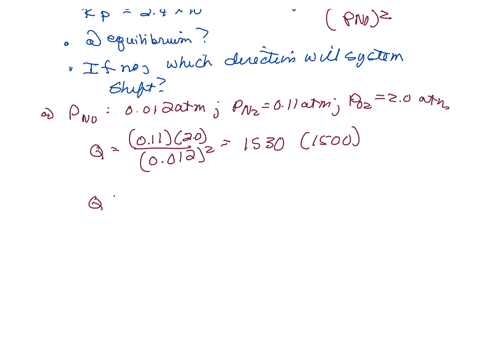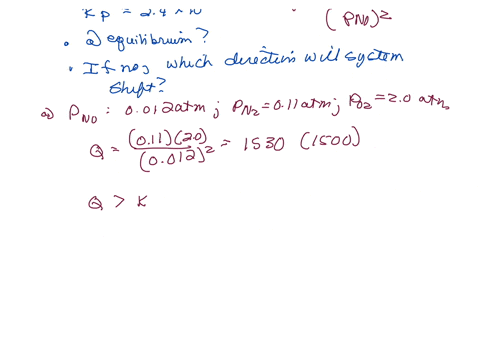
⏩SOLVED:The equilibrium constant Kp is 2.4 ×10^3 at a certain…

Consider the reaction: A(g) ⇌ B(g) + C(g) Find the equilibrium co

Solved Question 5 A reaction has an equilibrium constant of
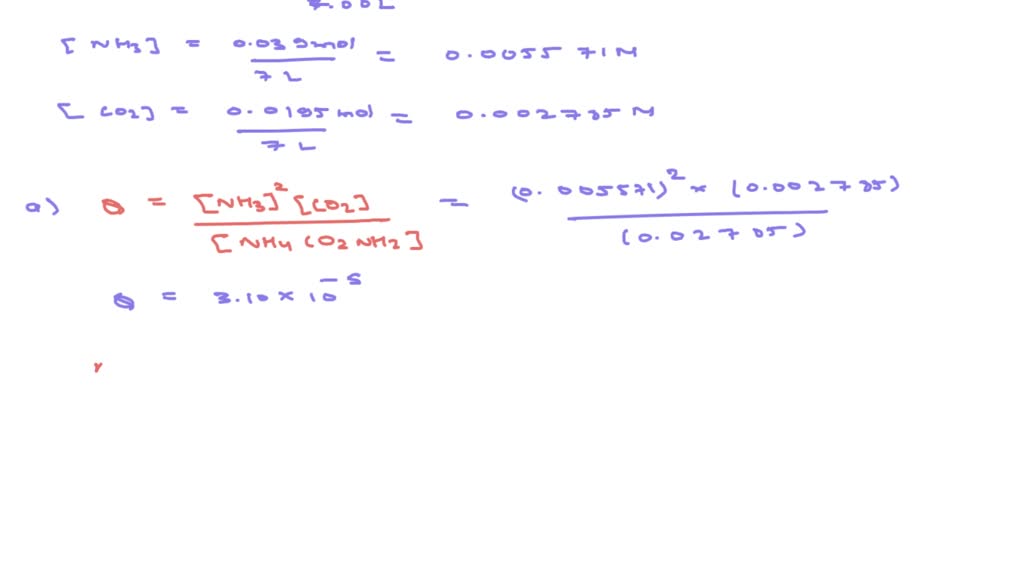
SOLVED: For the reaction below, the thermodynamic equilibrium constant is K = 1.33×10^(-2) at 45 °C. NH4CO2NH2(s) ⟶ 2NH3(g) + CO2(g) Suppose that 0.0085 moles of NH4CO2NH2, 0.017 moles of NH3, and