FDA warns on cybersecurity risk with Medtronic insulin pump
4.5 (658) · $ 23.00 · In stock

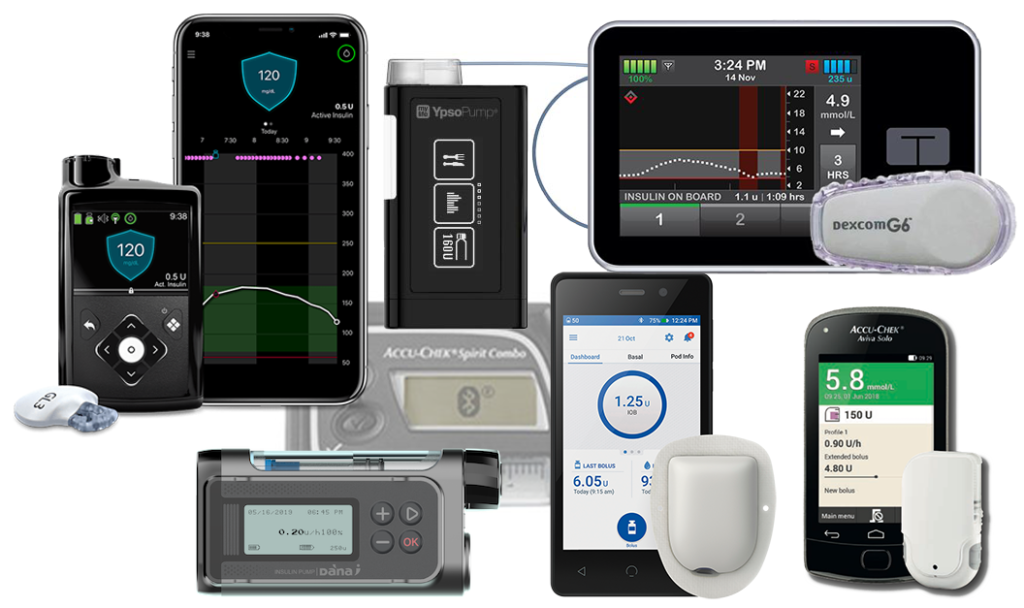
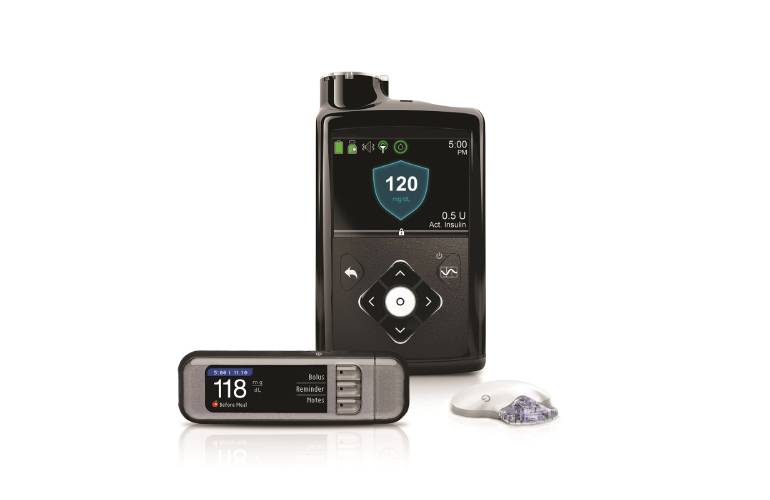
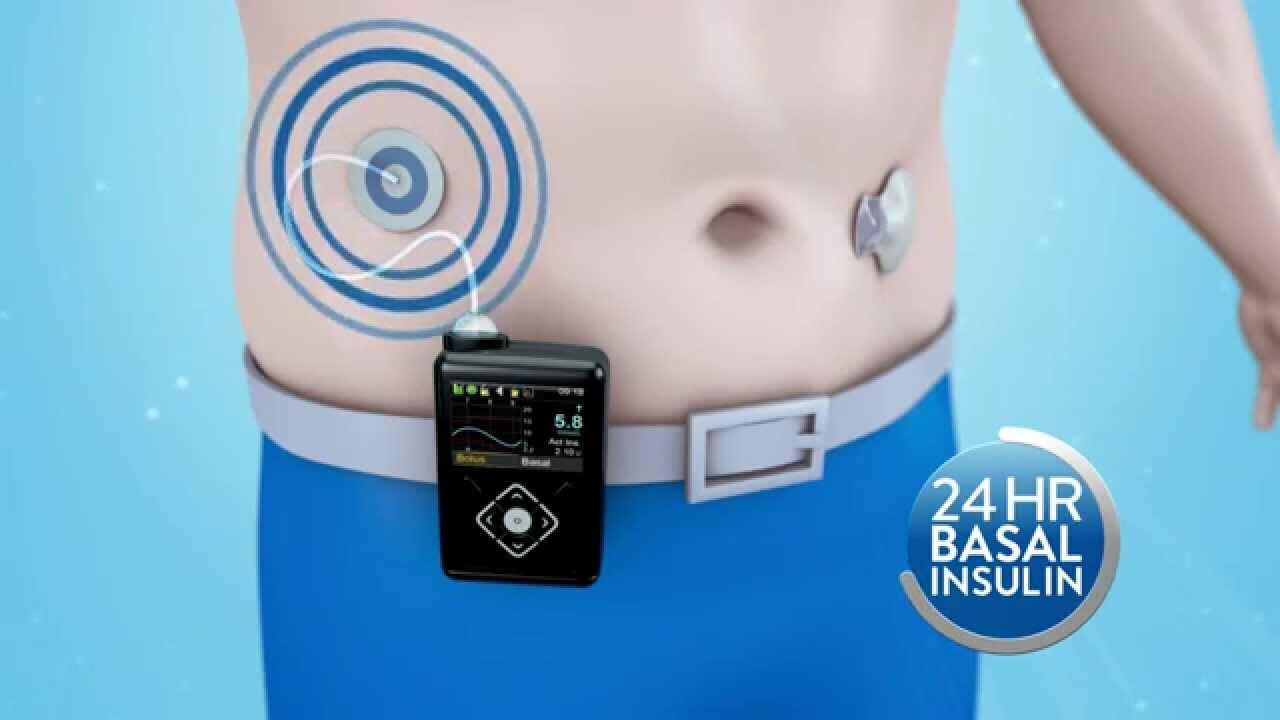
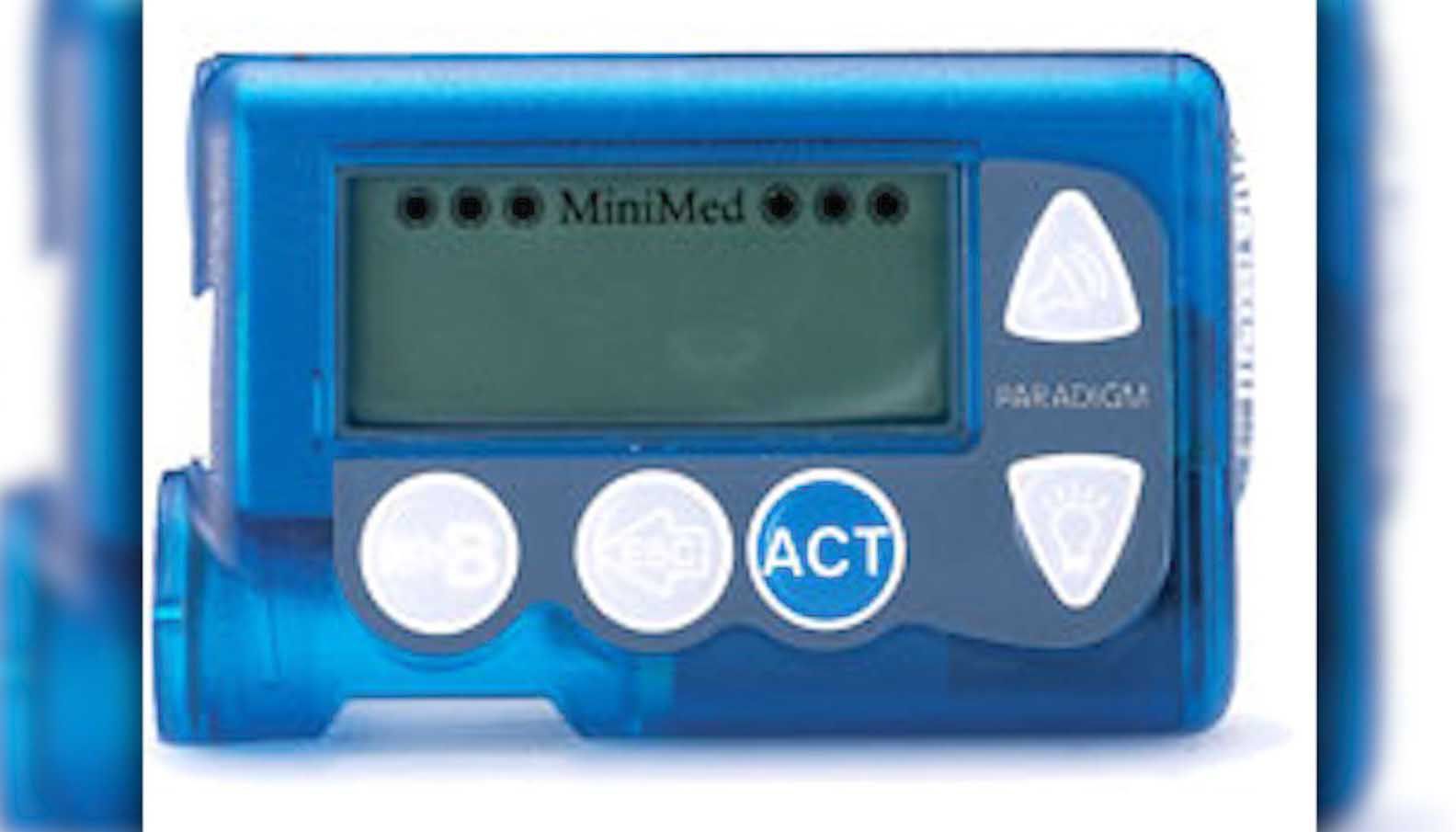
Medtronic's MiniMed 600 series insulin pumps potentially at risk of compromise, says FDA
FDA Warns Patients, Health Care Providers of issues with Medtronic Insulin Pumps
Medtronic recalls some insulin pumps as FDA warns of hacking risk

Medtronic MiniMed Insulin Pump Recall Leads to Lawsuits Filed

Medtronic MiniMed Insulin Infusion Pump Lawsuits – Parker Waichman LLP
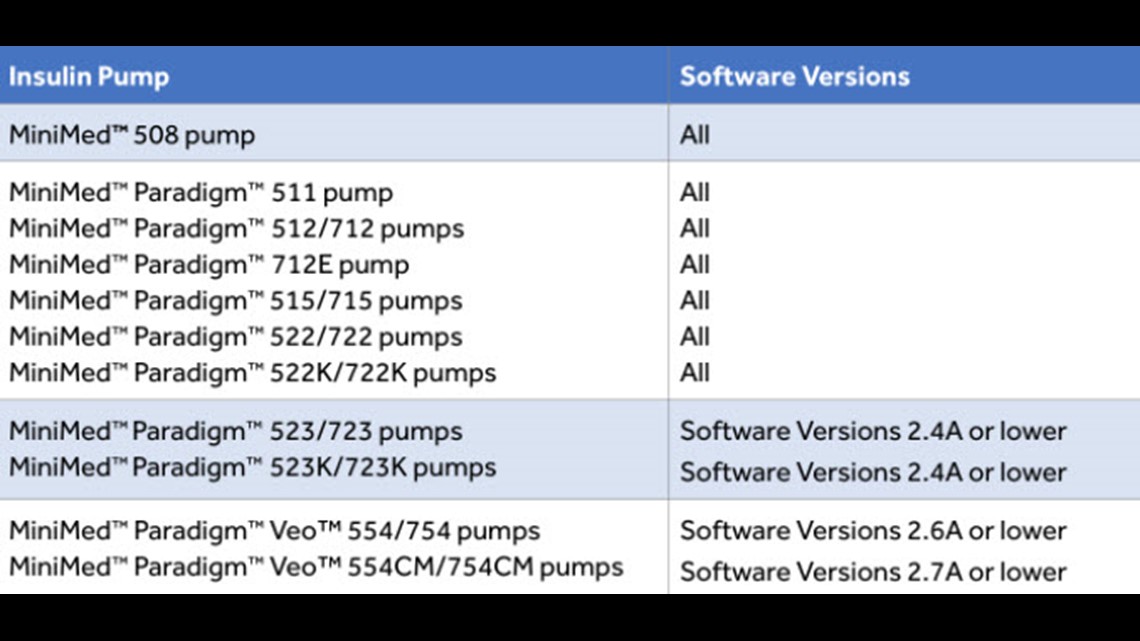
FDA warns patients and health care providers about potential cybersecurity concerns with certain Medtronic insulin pumps

Johnson & Johnson warns on low cyber risk with Animas OneTouch Ping insulin pump - MassDevice
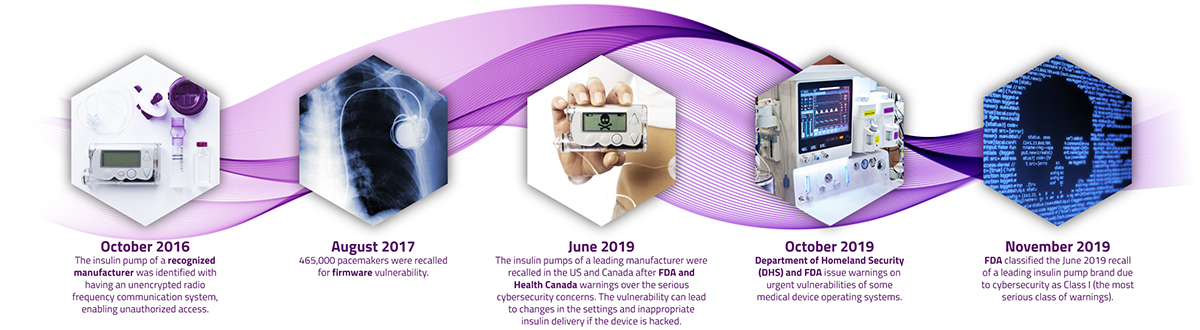
Column - Medical Device Cybersecurity in the Age of IoMT - MedTech Intelligence

FDA Says Medtronic Insulin Pumps Pose Cybersecurity Risk - WSJ

Insulin pumps recalled due to hacking risk

Medtronic MiniMed Insulin Pump Lawsuit - Seeger Weiss LLP

Medtronic recalls MiniMed insulin pumps as FDA warns about hacking risk
FDA warns on cybersecurity risk with Medtronic insulin pump

FDA warns of cybersecurity risk with certain Medtronic insulin pumps, ET HealthWorld

FDA Warns Of Cybersecurity Threat In Medtronic Insulin Pumps - BW Healthcare