- Home
- compressibility factor equation
- Which of the following statements is/are correct? (a) all real gases are less compressible
Which of the following statements is/are correct? (a) all real gases are less compressible
4.7 (557) · $ 22.99 · In stock
Which of the following statements is/are correct? (a) all real gases are less compressible than ideal gas at high pressures? (6) hydrogen and helium are more co
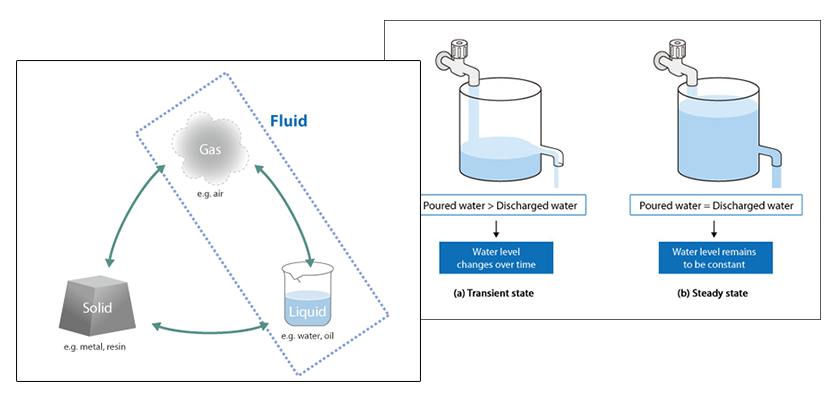
Basic Course of Thermo-Fluid Analysis 06: Chapter 3 Basics of Flow - 3.2.1 Compressible/incompressible fluids|List

For A Real Gas At 25∘C Temperature And High Pressure (99, 59% OFF

For A Real Gas At 25∘C Temperature And High Pressure (99, 59% OFF

The given graph represents the variation of Z (compressibility factor = \[\dfrac{{PV}}{{nRT}}\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.

Deviation Of Real Gas From Ideal Gas Behavior
Equations of Compressible and Incompressible Flow in Fluid Dynamics, System Analysis Blog
How can a gas be ideal at a high pressure and low temperature? - Quora
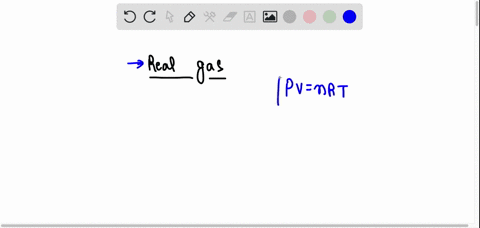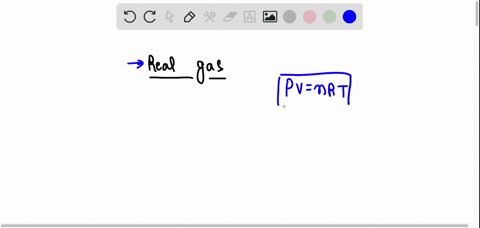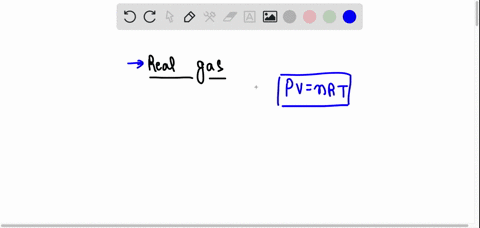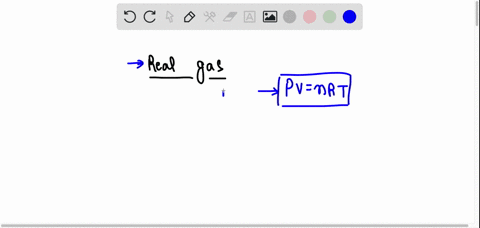
SOLVED: The behavior of real gases is different from that predicted for ideal gases. Which of the following statements about real gases is not correct? A. Gas molecules have potential energy. B. Forces between gas molecules are always negligible. C. Gas
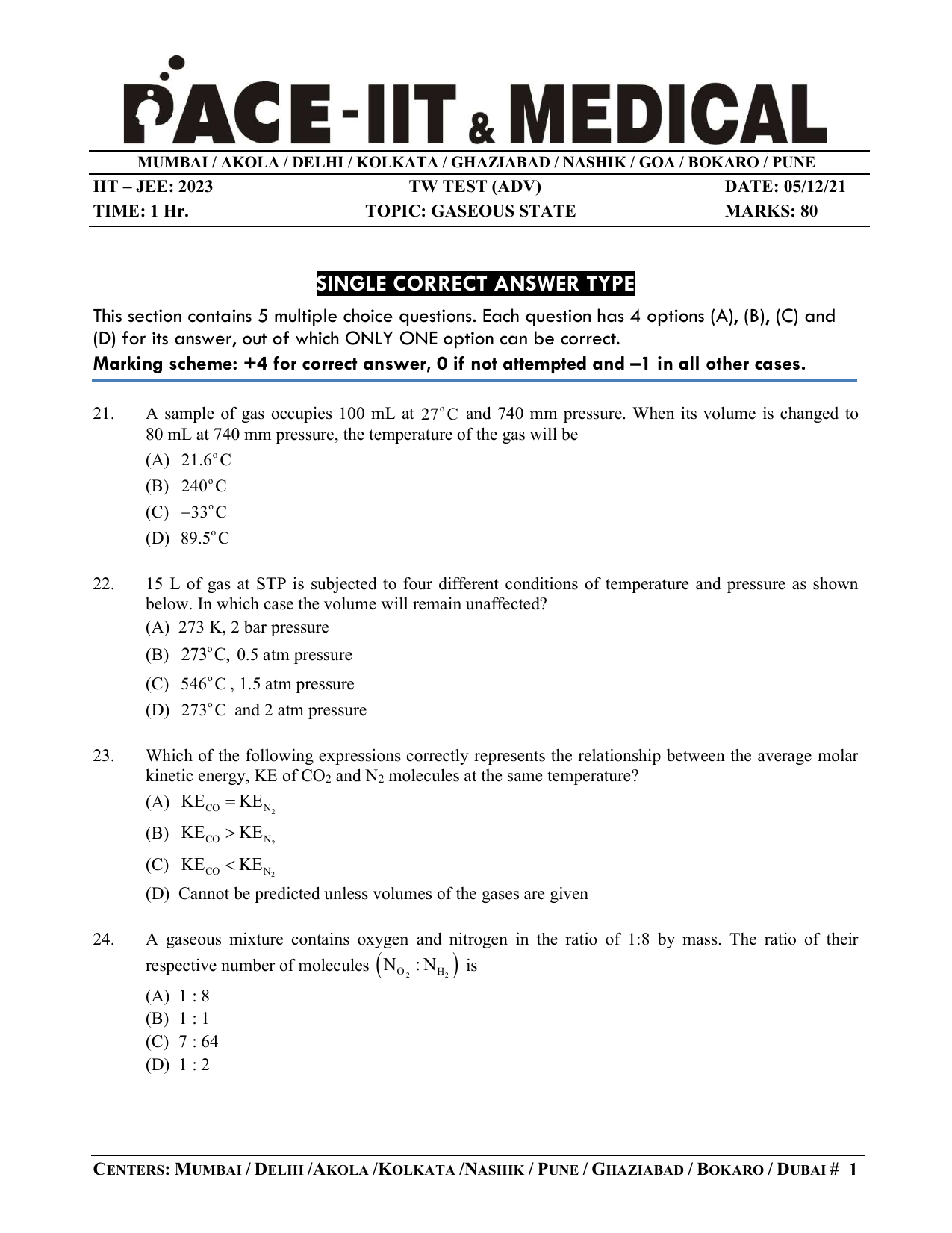
Gaseous State (ADV) Question Paper

Van der Waals equation - Wikipedia
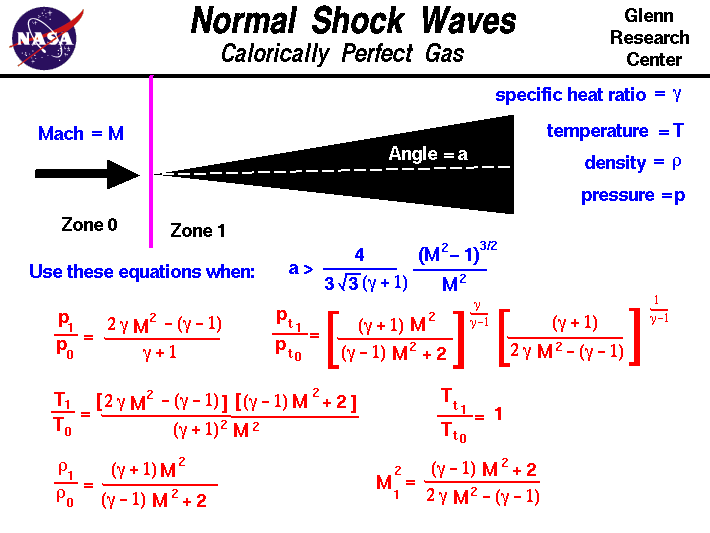
Normal Shock Wave Equations

Compressibility factor (z): real gases deviate from ideal behav-Turito

Which of the following statement is/are correct ? a.All real gases are less compressible than ideal gases at











